29 Cu (Copper)

It is reddish and takes on a bright metallic luster, It is malleable, ductile, and a second high conductor of heat and electricity (first is Silver in electrical conductivity).
It reflects red and orange light and absorbs other frequencies in the visible spectrum.
ACopper has low reactive in moist air, where it slowly forms a greenish surface film called patina (this coating protects the metal from further attack).
Identity
CAS Number: CAS7440-50-8
CID Number: CID23978
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSGL5325000
CONTENT INDEX
Basic Properties of Copper
Appearance: red-orange Metallic luster
Mass Number: 64
Standard Atomic weight: 63.546 g/mol
Atomic number (Z): 29
Electrons: 29
Protons: 29
Neutrons: 35
Period: 4
Group: 11
Block: d
Element category: Transition metal
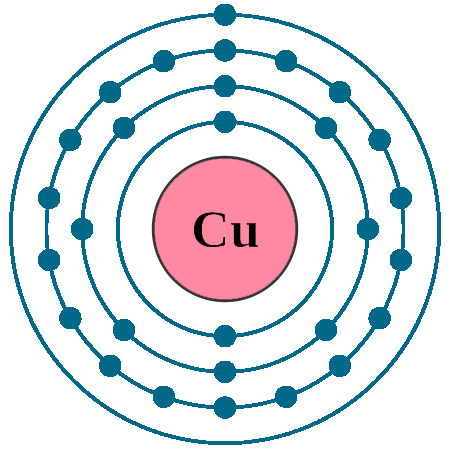
Electrons per shell: K2, L8, M18, N1
Electron configuration: 1s22s22p63s23p63d104s1
Thermal Properties of Copper
Phase: Solid
Melting point: 1358 K (1085 oC, 1985 oF)
Boiling point: 2835 K (2562 oC, 4643 oF)
Debye temperature: 315 K (41.85 oC, 107.33 oF)
Fusion heat: 13.26 kJ/mol
Vaporization heat: 300.4 kJ/mol
Specific heat: 384.4 J/(kg K)
Molar heat capacity: 24.440 J/(mol.K)
Thermal expansion: 16.5 μm/(m∙K)
Thermal conductivity: 401 W/(m∙K)
Electrical properties of Copper
Electrical conductivity: 59×106 S/m
A Electrical resistivity: 16.78 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Copper
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): –5.46×10-6 cm3/mol
Volume magnetic susceptibility: -0.00000963
Mass magnetic susceptibility: -1.08×10-9 m3/kg
Molar magnetic susceptibility: -0.0686×10-9 m3/mol
Physical Properties of Copper
Density: 8.96 g/cm3 (In solid) 8.02 g/cm3 (In Liquid at M.P)
Molar volume: 0.000007092 m3/mol
Young’s modulus: 110-128 GPa
Shear modulus: 48 GPa
Mohs Hardness: 3.0
Bulk modulus: 140 GPa
Poisson ratio: 0.34
Vicker hardness: 342-369 MPa
Brinell hardness: 235-878 MPa
Sound Speed: 3810 m/s
Atomic Properties of Copper
Oxidation states: -2, +1, +2, +3, +4
Valence Electrons: 3d10 4s1
Ion charge: Cu2+ Cu+
The ionization potential of an atom: 7.69
Ionization energies: 1st: 745.5 kJ.mol 2nd: 1957.9 kJ/mol 3rd: 3555 kJ/mol
Ionic radius: 73 pm
Atomic radius: empirical: 128 pm
Van der Waals: 140 Pm
Covalent radius: 132±4 pm
Filling Orbital: 3d10
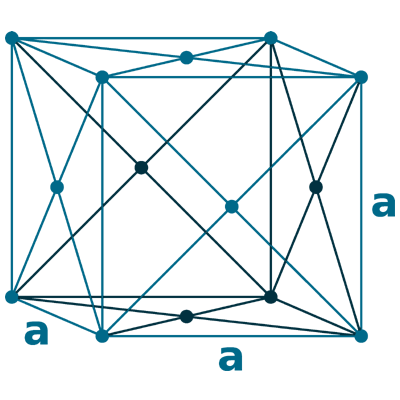
Crystal structure: Face centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 361.5, 361.5, 361.5 pm
Grid parameters: a=3.615 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Copper
Electronegativity: pauling scale: 1.90
Valence: +2
Electron affinity: 118.4 kJ/mol
Nuclear Properties of Copper
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 3.78
Neutron Mass Absorption: 0.0021
Isotopes: 63Cu 64Cu 65Cu 67Cu
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 63Cu | 69.15 | 62.930 | Stable |
| 64Cu | Syn | – | 12.70 h |
| 65Cu | 30.85 | 64.928 | Stable |
| 67Cu | Syn | – | 61.83 h |
Chemical Reactions of Copper
The metal is stable in air under normal conditions. When heated tored hot, It reacts with oxygen, and forming Cu2O.
4 Cu (s) + O2 (g) → 2 Cu2O (s)
Reacts with Halogens, and forming:
Cu (s) + F2 (g) → CuF2 (s) [white] (Copper (ll) fluoride)
Cu (s) + Cl2 (g) → CuCl3 (s) [yelow-brown] (Copper (ll) chloride)
Cu (s) + Br2 (g) → CuBr3 (s) [black] (Copper (ll) bromide)
The metal dissolves in hot concentrated sulphuric acid (H2SO4) , and forming Cu(II) ions and hydrogen. Aquated solution of Cu(II) is present as the complex ion [Cu(H2O)6]2+.
Cu (s) + H2SO4 (aq) → Cu2+ (aq) + SO42- (aq) + H2 (g)
Metal also dissolves in dilute or concentrated nitric acid, HNO3.
3 Cu (s0 + 2 NO3– (aq) → 3 Cu2+ (aq) + 2 NO (g) + 4 H2O (I)
Production:
Cu2S, is roasted to convert all sulfides into oxides:
2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
cuprous oxide is converted to blister copper upon heating:
2 Cu2O → 4 Cu + O2
Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining, and In resulting Pure copper is produce.
Cu2+ + 2 e– → Cu
Copper History
Naming: After Cyprus, principal mining place in Roman era (Cyprium)
Discovery: Middle East (9000 BC)
Copper Uses
Most copper is used in electrical industry (such as wiring and motors, 60%), In Construction (such as roofing and plumbing, 20%), in industrial machinery (such as heat exchangers,15%) and alloys (5%).
Traditionally it has been one of the metals used to make coins, and all US coins are now copper alloys.
The main long established copper alloys are Brass (a copper-zinc alloy), Bronze (88% Cu & 12%Sn), Cupro-nickel (copper-nickel-iron) alloy was the preferred metal for low-denomination coins, Copper-Tin-Zinc (55% Cu, 30%Sn, 15% Zn) alloy was strong enough to make guns and cannons, and was known as gun metal.
Copper sulfate is widely used as an agricultural poison and as an algicide in water purification.
Copper compounds, such as Fehling’s solution, are used in chemical tests for sugar detection.
Biological role of Copper
ACopper found in many kinds of food, in drinking water, and in air.
It is an essential element in plants and animals, An adult human needs around 1.4 to 1.9 mg (milligrams) of copper a day, to help enzymes transfer energy in cells, and human absorb eminent quantities of copper each day by eating, drinking and breathing.
Although humans can handle proportionally large concentrations of copper, Because excess copper could be toxic, which cause eminent health problems.
Genetic diseases, such as Wilson’s disease and Menkes’ disease, that can affect the body’s ability to use copper properly.
Intentionally high uptakes of copper may cause liver and kidney damage and even death.
Unlike mammals, which use iron (in haemoglobin) to transport oxygen around their bodies, some crustaceans use copper complexes.
Abundance of Copper
ACopper metal does occur naturally, and is found in many minerals such as cuprite (Cu2O), malachite (Cu2CO3(OH)2), azurite (Cu3(CO3)2(OH)2), chalcopyrite (CuFeS2), and bornite (Cu5FeS4).
The most important copper ores are the sulfides, the oxides, and carbonates.
It is obtained from these ores and minerals by smelting, leaching and electrolysis.
High-purity copper (99.999+ %) is available commercially.
Annual world wide production is around 17,000,000 tons.
60×10-7% (In Universe)
0.011% (In Meteorites)
7×10-5% (In Sun)
0.0068% (In Earth’s Crust)
3×10-7% (In Oceans)
0.0001% (In Humans)





World’s Top 3 producers of Copper
1) Chile
2) Peru
3) China
World’s Top 3 Reserve holders of Copper
1) Chile
2) Peru
3) Australia
#Copper


