30 Zn (Zinc)

Zinc is a lustrous, bluish-white metal. It is brittle and crystalline at ordinary temperatures, but it becomes ductile and malleable at 100°C and 150°C.
It is a fair conductor of electricity, and burns in air at high red heat temperature with rise of white clouds of the oxide.
Both zinc & zirconium is not ferromagnetic, But ZrZn2 exhibits ferromagnetism at temperatures below 35°K (-238.15 oC).
It is a quite reactive metal that combines with oxygen and other non-metals, and reacts with dilute acids to release hydrogen.



Identity
CAS Number: CAS7440-66-6
CID Number: CID23994
DOT Hazard Class: 4.3
DOT Number: 1436
RTECS Number: RTECSZG8600000
CONTENT INDEX
Basic Properties of Zinc
Appearance: Silver-gray
Mass Number: 65
Standard Atomic weight: 65.38 g/mol
Atomic number (Z): 30
Electrons: 30
Protons: 30
Neutrons: 35
Period: 4
Group: 12
Block: d
Element category: Transition metal
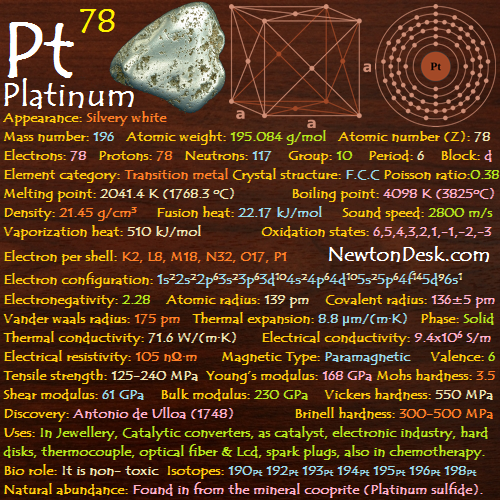
Electrons per shell: K2, L8, M18, N2
Electron configuration: 1s22s22p63s23p63d104s2
Thermal Properties of Zinc
Phase: Solid
Melting point: 692 K (419.53 oC, 787.15 oF)
Boiling point: 1180 K (907 oC, 1665 oF)
Debye temperature: 234 K (-39.15 oC, -38.47 oF)
Fusion heat: 7.32 kJ/mol
Vaporization heat: 115 kJ/mol
Specific heat: 399 J/(kg K)
Molar heat capacity: 25.470 J/(mol.K)
Thermal expansion: 30.2 μm/(m∙K)
Thermal conductivity: 116 W/(m∙K)
Electrical properties of Zinc
Electrical conductivity: 17×106 S/m
A Electrical resistivity: 59 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.85 K (-272.3 oC, -458.14 oF)
Magnetic Properties of Zinc
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -11.4×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000158
Mass magnetic susceptibility: -2.21×10-9 m3/kg
Molar magnetic susceptibility: -0.145×10-9 m3/mol
Physical Properties of Zinc
Density: 7.14 g/cm3 (In solid) 6.57 g/cm3 (In Liquid)
Molar volume: 0.000009157 m3/mol
Refractive index: 1.00205 (Ratio of the velocity of light in a vacuum to its velocity in a specified medium)
Young’s modulus: 108 GPa
Shear modulus: 43 GPa
Mohs Hardness: 2.5
Bulk modulus: 70 GPa
Poisson ratio: 0.25
Brinell hardness: 327-412 MPa
Sound Speed: 3850 m/s
Atomic Properties of Zinc
Oxidation states: –2, 0, +1, +2
Valence Electrons: 4s2
Ion charge: Zn2+
The ionization potential of an atom: 9.35
Ionization energies: 1st: 906.4 kJ.mol 2nd: 1733.3 kJ/mol 3rd: 3833 kJ/mol
Ionic radius: 74 pm
Atomic radius: empirical: 134 pm
Van der Waals radius: 139 Pm
Covalent radius: 122±4 pm
Filling Orbital: 3d10
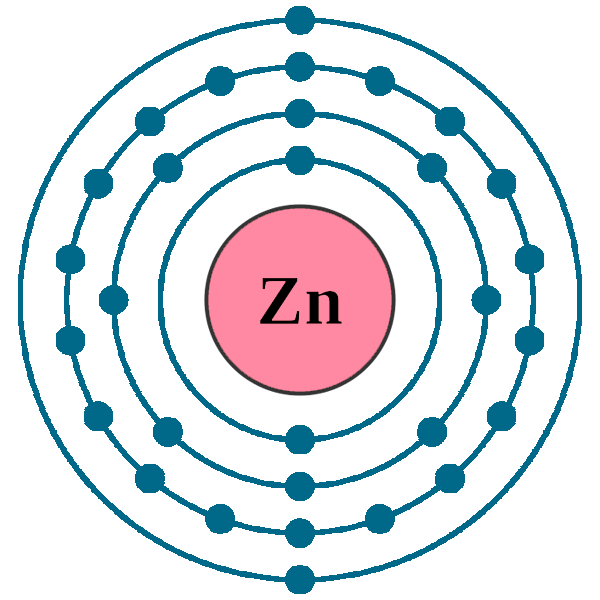
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 266.48, 266.48, 494.68 pm
Grid parameters: a=2.6648 Å c=4.9468 Å
Attitude c/a: 1.856
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Zinc
Electronegativity: pauling scale: 1.65
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Zinc
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 1.1
Neutron Mass Absorption: 0.00055
Isotopes: 64Zn 65Zn 66Zn 67Zn 68Zn 69Zn 69mZn 70Zn 71Zn 71mZn 72Zn
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 64Zn | 49.2 | 63.929 | Stable |
| 65Zn | Syn | – | 244 d |
| 66Zn | 27.7 | 65.926 | Stable |
| 67Zn | 4.0 | 66.927 | Stable |
| 68Zn | 18.5 | 67.925 | Stable |
| 69Zn | Syn | – | 56 min |
| 69mZn | Syn | – | 13.8 h |
| 70Zn | 0.6 | 69.925 | Stable |
| 71Zn | Syn | – | 2.4 min |
| 71mZn | Syn | – | 4 d |
| 72Zn | Syn | – | 46.5 h |
Chemical Reactions of Zinc
Reacts with oxygen in moist air and Burns in air to form zinc (II) oxide, and a material that turns white to yellow on prolonged heating:
2 Zn (s) + O2 (g) → 2 ZnO (s) [white]
Zinc doesn’t react with water, but reduce stem at high temperatures:
Zn (s) + H2O (g) → ZnO (s) + H2 (g), ∆H = -106.4 KJ
Reacts with gaseous bromine & Iodine:
Zn (s) + Br2 (g) → ZnBr3 (s) [white] (Zinc (ll) bromide)
Zn (s) + I2 (g) → ZnI3 (s) [white] (Zinc (ll) iodide)
The metal dissolves slowly in dilute sulphuric acid (H2SO4) to form Zn(II) ions and hydrogen (H2), aqueous solution the Zn(II) ion is present as the complex ion [Zn(H2O)6]2+:
Zn (s) + H2SO4 (aq) → Zn2+ (aq) + SO42- (aq) + H2 (g)
It isdissolved in concentrated nitric acid, HNO3:
Zn (s) + 4 HNO3 (aq) → Zn(NO3)2 (aq) + 2 NO2 (g) + 2 H2O (l)
Production
Roasting converts the zinc sulfide concentrate to zinc oxide:
2 ZnS + 3 O2 → 2 ZnO + 2 SO2
Zinc oxide with carbon or carbon monoxide at 950 °C (1,740 °F) into the metal:
2 ZnO + C → 2 Zn + CO2
ZnO + CO → Zn + CO2
The zinc vapor is collected in a condenser.
In Electrowinning process, zinc is leached from the ore concentrate by sulfuric acid (H2SO4):
ZnO + H2SO4 → ZnSO4 + H2O
Finally, the zinc is reduced by electrolysis:
2 ZnSO4 + 2 H2O → 2 Zn + 2 H2SO4 + O2
Zinc History
Naming: German: zink (German for tin)
Recognized as a unique metal by: Rasaratna Samuccaya (800)
Discovery: Indian metallurgists (before 1000 BC)
First isolation: Andreas Sigismund Marggraf (1746)
Zinc Uses
It is principally used to galvanise other metals, such as Iron (to prevent rusting or corrosion).
Most of metallic zinc goes into galvanizing steel, which is used for car bodies, street lamp posts, safety barriers and suspension bridges.
It is used for the negative plates in some electric batteries and for roofing and gutters in building construction.
It is also important in the preparation of certain alloys, Brass (Cu3Zn2) , typewriter metal, nickel silver (60% Cu, 20% Ni & 20% Zn), commercial bronze (90% Cu & 10% Zn), German silver, spring bronze, soft solder, and aluminum solder are some of the more important alloys.
Large quantities of zinc are used to produce die-castings, which are important in the automobile, electrical and hardware industries.
An alloy called Prestal(R) (78 %Zn & 22% Al) is reported to be almost as strong as steel and as easy to mold as plastic, It can be molded into form using inexpensive ceramics or cement die casts.
Zinc oxide (ZnO) is very useful and is widely used in the manufacture of very many products such as paints (as a white pigment), rubber (as an activator), pharmaceuticals, batteries, cosmetics, plastics, soaps, inks, textiles, electrical equipment etc..
In Rubber production its used as a catalyst during manufacture and as a heat disperser in the final product.
Lithopone is a mixture of zinc sulfide (ZnS) and barium sulfate (BaSo4), is an important pigment.
Zinc sulfide is used in making luminous dials, X-ray and TV screens, and fluorescent lights.
Biological role of Zinc
Zinc is not considered to be toxic, it is essential for all living things.
The average human body contains about 2.5 grams and intakes about 15 mg (milligrams) per day.
Some foods have above average levels of zinc, including herring (forage fish), lamb (meat of young domestic sheep), beef (meat from cattle), sunflower seeds and cheese.
Zinc can be carcinogenic (A substance that promotes the formation of cancer) in excess.
If freshly formed ZnO (zinc oxide) is inhaled a disorder known as ‘oxide shakes’ or ‘zinc chills’ sometimes occurs, mostly it is formed during the welding of galvanized materials.
Abundance of Zinc
Zinc is found in several ores. The principal ores of zinc are sphalerite (sulfide) (Zn,Fe)S, calamine (silicate), smithsonite (carbonate), and franklinite (zinc, manganese, iron oxide). Commercially, zinc is obtained by concentrating and roasting its ores to form the oxide and then reducing the oxide to zinc by heating with carbon or by electrolysis.
Annual world wide production is around 14,000,000 tons.
0.00003% (In Universe)
0.018% (In Meteorites)
0.0002% (In Sun)
0.0078% (In Earth’s Crust)
5×10-7% (In Oceans)
0.0033% (In Humans)




World’s Top 3 producers of Zinc
1) China
2) Australia
3) Peru
World’s Top 3 Reserve holders of Zinc
1) Australia
2) China
3) Peru
#Zinc