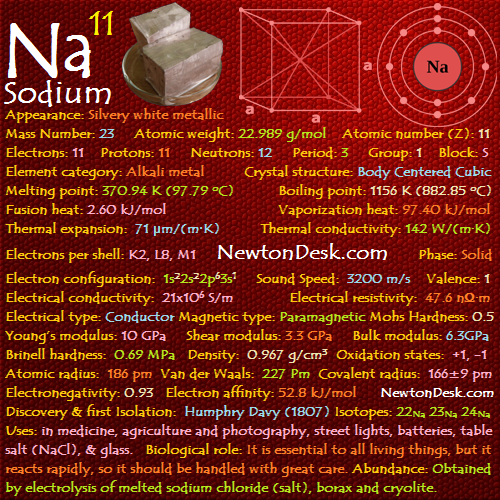
11 Na (Sodium Element)
Sodium is a soft, reactive, bright-silvery metal with the low melting point, and it is not found free in nature.
Sodium metal reacts with air, and looses its silvery appearance and acquires an opaque (not transparent) grey color due to the formation of a sodium oxide (Na2O) coating.
Sodium reacts readily with water, snow & ice, to produce sodium hydroxide (NaOH) and hydrogen.
It may or may not ignite spontaneously on water, It all depends on the amount of oxide and metal exposed to the water,
A tiny amount of metal floats on water, but not only high, even normal quantity of metal ignite spontaneously on water.
Sodium doesn’t react with nitrogen, even not react at very high temperatures, but it can react with ammonia (NH3) to form sodium amide (NaNH2).
Sodium & hydrogen reacts above 200ºC to form sodium hydride (NaH).
Sodium rarely reacts with carbon, but it does react with halogens (F, Cl, Br, I).
It also reacts with various metallic halides to form the metal & sodium chloride (NaCl).
Sodium doesn’t react with paraffinic hydrocarbons (alkane), But it forms addition compounds with naphthalene (C10H8), aryl alkenes, and other aromatic polycyclic compounds.
That’s why the metal stores in Kerosene (a complex mixture of Naphthenes (40.8%), Paraffins (55.3%), and Aromatic hydrocarbons (3.9%).
Sodium reacts with alcohols is similar, as reacts with water, But with alcohol, it reacts slowly.

Identity
CAS Number: CAS7440-23-5
CID Number: CID5360545
DOT Hazard Class: 4.3
DOT Number: 1428
RTECS Number: RTECSVY0686000
Basic Properties of Sodium
Pronunciation: S-au-di-am
Appearance: Silvery white metallic
Mass Number: 23
Standard Atomic weight: 22.989 g/mol
Atomic number (Z): 11
Electrons: 11
Protons: 11
Neutrons: 12
Period: 3
Group: 1
Block: s
Element category: Alkali metal
Electrons per shell: K2, L8, M1
Electron configuration: 1s22s22p63s1

CONTENT INDEX
Thermal Properties of Sodium
Phase: Solid
Melting point: 370.94 K (97.79 oC, 208.02 oF)
Boiling point: 1156 K (882.85 oC, 1621.14 oF)
Debye temperature: 150 K (-123.15 oC, -189.67 oF)
Fusion heat: 2.60 kJ/mol
Vaporization heat: 97.40 kJ/mol
Critical Temperature: 2571 K (2297.85 oC, 4168.13 oF)
Critical Pressure: 35 MPa (345.423 Atm)
Specific heat: 1230 J/(kg K)
Molar heat capacity: 28.230 J/(mol.K)
Thermal expansion: 71 μm/(m∙K)
Thermal conductivity: 142 W/(m∙K)
Electrical properties of Sodium
Electrical conductivity: 21×106 S/m
A Electrical resistivity: 47.6 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Sodium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +16×10-6 cm3/mol
Volume magnetic susceptibility: 0.0000062
Mass magnetic susceptibility: 6.4×10-9 m3/kg
Molar magnetic susceptibility: 0.2×10-9 m3/mol
Physical Properties of Sodium
Density: 0.967 g/cm3 (In solid) 0.928 g/cm3 (In Liquid at M.P)
Molar volume: 0.00002375 m3/mol
Young’s modulus: 10 GPa
Shear modulus: 3.3 GPa
Mohs Hardness: 0.5
Bulk modulus: 6.3GPa
Vicker hardness: MPa
Brinell hardness: 0.69 MPa
Sound Speed: 3200 m/s
Atomic Properties of Sodium
Oxidation states: +1, -1
Valence Electrons: 3s1
Ion charge: Na+
Ionization potential of an atom: 5.12
Ionization energies: 1st: 495.70 kJ.mol 2nd: 4561.5 kJ/mol 3rd: 6910 kJ/mol
Ionic radius: 102 pm
Atomic radius: 186 pm (empirical)
Van der Waals: 227 Pm
Covalent radius: 166±9 pm
Filling Orbital: 3s1
Crystal structure: Body centered cubic (B.C.C)
Lattice angles: π/2, π/2, π/2
Lattice constant: 428.20, 428.20, 428.20 pm
Grid parameters: a=4.282 Å
Space Group Name: lm_3m
Space Group Number: 229

Reactivity of Sodium
Electronegativity: 0.93 (pauling scale)
Valence: 1
Electron affinity: 52.8 kJ/mol
Nuclear Properties of Sodium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 0.53
Neutron Mass Absorption: 0.0007
Isotopes: 22Na 23Na 24Na
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 22Na | Trace | – | 2.601 y |
| 23Na | 100 | 22.990 | Stable |
| 24Na | Trace | – | 14.95 h |
Chemical Reactions of Sodium
Sodium reacts with air, and forming sodium oxide (Na2O), But if sodium burned, than it forms sodium peroxide (Na2O2) with some sodium oxide:
4 Na (s) + O2 (g) → 2 Na2O (s)
2 Na (s) + O2 (g) → Na2O2 (s)
The metal reacts violently with water, where it forms a colorless solution of sodium hydroxide (NaOH) & Haydrogen (H2).
It is very exothermic reaction, where during the reaction, the metal become so hot, and due to high temprature, it catches fire and burn with a orange color:
2 Na (s) + 2 H2O → 2 NaOH (aq) + H2 (g)
OR
2 Na (s) + 2 H2O (l) → 2 Na+ (aq) + 2 OH‑ (aq) + H2 (g)
It reacts vigorously with Halogens, and forming the corresponding sodium (I) halides:
2 Na (s) + F2 (g) → 2 NaF (s) (Sodium fluoride)
2 Na (s) + Cl2 (g) → 2 NaCl (s) (Sodium chloride)
2 Na (s) + Br2 (g) → 2 NaBr (s) (Sodium bromide)
2 Na (s) + I2 (g) → 2 NaI (s) (Sodium iodide)
The metal dissolves readily in dilute sulphuric acid (H2SO4), and forming the solutions containing the aquated Na (I) ion together with hydrogen gas (H2).
2 Na (s) + H2SO4 (aq) → 2 Na+ (aq) + SO42- (aq) + H2 (g)
When Sodium reacts with hydrogen, it forms sodium hydride:
2 Na (s) + H2 (g) → 2 NaH (s)
It reacts with sulfur, and forming sodium sulfide:
16 Na (s) + S8 (s) → 8 Na2S (s)
Reacts with Phosphorus, It forms sodium phosphide:
12 Na (s) + P4 (s) → 4 Na4P (s)
Sodium History
Naming: From soda (Na2CO3); Na from Latin natrium
Discovery & first Isolation: Humphry Davy (1807)
Sodium Uses
Sodium is used as a reagent (a mixture or substance for use in chemical analysis or other reactions) in the chemicals industry and as a heat exchanger in some nuclear reactors.
Sodium salts have more uses than sodium metal, where the most common compound of sodium is sodium chloride (salt, NaCl), which is added in food, and used to de-ice roads in winter, and also used as a feedstock (raw material) for the chemical industry.
Ionized Sodium is used in sodium vapour lamps (street lights), and wavelength of its produce light is near about 589 nm.

A Sodium hypochlorite (Bleach, NaOCl) is effectively used for water purification, bleaching, surface purification, odor removal, water disinfection etc..
Sodium carbonate (washing soda, Na2CO3) is also a useful sodium salt, that is used to make Glass, Rayon, Paper, Soaps, and Detergents, and also used as a water softener.
Sodium bicarbonate (baking soda, NaHCO3) reduces acid of the stomach, so, It is used as an antacid to treat heartburn, indigestion, & upset stomach.
A Sodium borate (borax, Na2B4O7) is used to make buffer solution (aqueous solution) as an insecticide, as an Anti-fungal compound for fiberglass, as a Fire retardant, as a flux in metallurgy, texturing agent in Cooking, Neutron-capture shields for radioactive sources etc..
Sodium benzoate (NaC7H5O2) is widely used in antifungal preservation of food (Such as soft drinks, pickles, fruit pies, jams etc..) with an E number (E211), also used in cosmetics.
Sodium thiosulfate (hypo, Na2S2O3) is used as an Antidote to Cyanide Poisoning.
Sodium nitrate (Chile saltpeter, NaNO3) is an odorless, crystalline, colorless compound, that is used to make potassium nitrate, in solid propellants, in explosives, in fertilizers, in the production of high-strength glass etc..
Metallic form of sodium (sodium metal) is essential in the manufacture of esters and in the preparation of organic compounds (contains carbon).
It may be used to improve the structure of certain alloys, to purify molten metals, & to descale metal (cleaning process to provide smooth surface finish).
It is alloyed with potassium (NaK) to make an important heat transfer agent.
Biological role of Sodium
Humans have already known from prehistoric times, that Sodium is essential to all living things.
Our bodies already contain around 90-110 grams of sodium, and the average person daily intake around 10-20 grams of salt, but all we really need is around 3 grams/day, which can get from food without adding any extra.
Extra sodium may damage our kidneys and contribute to high blood pressure, but Sodium is also important for many different functions of the human body, such as it helps cells to transmit nerve signals and regulate water levels in blood & tissues.
Contact of sodium metal with water causes the formation of sodium hydroxide (NaOH) fumes, which is highly irritating to eyes, nose, throat, & skin.
Sodium metal cannot be maintained in an inert atmosphere, water, and other substances, because it reacts rapidly, that’s why it should be handled with great care.
Abundance of Sodium
Sodium is the 6th most abundant element in the Earth crust, and it is also most abundant from other alkali groups of metals.
Sodium Salts are found in nature, such as Sodium carbonate (Soda ash or trona, Na2CO3), Sodium bicarbonate (baking soda, NaHCO3), Sodium borate (borax, Na2B4O7), Sodium chloride (rock salt or halite, NaCl), Sodium thiosulfate (hypo, Na2S2O3•5H2O), Sodium nitrate (Chile saltpeter, NaNO3), and Sodium sulfate (Na2SO4).
The most common and important compound of sodium is sodium chloride (NaCl), which is more dense soluble salt that has been leached into the oceans over the lifetime of the planet, but many salt beds or salt lakes are found, where the ancient seas have been evaporated.
It is also found in some minerals including Niter, Zeolite (NaAlSi2O6-H2O), Amphibole, Cryolite (Na3AlF6), & Sodalite (Na8Al6Si6O24Cl2).
Sodium is so reactive, that’s why it is not found as the metal in nature.
The metal is commercially produced by electrolysis of absolutely dry molten (fused) sodium chloride (NaCl).
This method is much cheaper than the process of electrolyzing sodium hydroxide (caustic soda, NaOH), which was used several years ago.



Annual world wide production of metallic sodium is around 150,000 tonnes.
0.002% (In Universe)
0.55% (In Meteorites)
0.004% (In Sun)
2.3% (In Earth’s Crust)
1.1% (In Oceans)
0.14% (In Humans)
World’s Top 3 producers of Sodium
1) China
2) India
3) USA
Sodium Price
Pure (99.5%) metallic sodium price is around $100-$150 per KG (KiloGram)
#sodium


