71 Lu (Lutetium)


Identity
CAS Number: 7439-94-3
CID Number: CID23929
CONTENT INDEX
Basic properties of Lutetium
Appearance: Hard, Silvery white
Mass number: 175
Standard Atomic weight: 174.9668 g/mol
Atomic number: 71
Electrons: 71
Protons: 71
Neutrons: 104
Period: 6
Block: f
Element category: Lanthanide
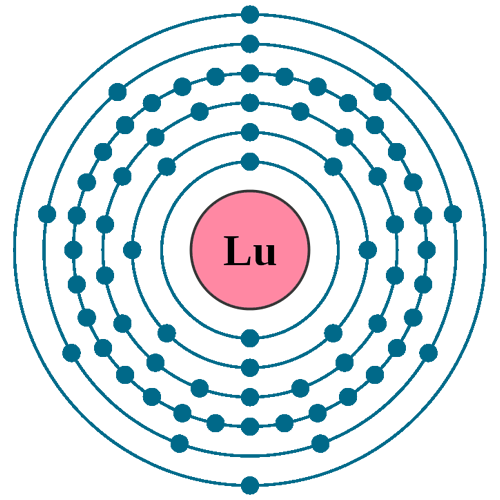
Electrons per shell: K2, L8, M18, N32, O9, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d16s2
Thermodynamic Property of Lutetium
Phase: Solid
Melting point: 1925 K (1652 oC, 3006 oF)
Boiling point: 3675 K (3402 oC, 6156 oF)
Fusion Heat: ca. 22 kJ/mol
Vaporization Heat: 414 kJ/mol
Molar heat capacity: 26.86 J/(mol∙K)
Thermal expansion: poly: 9.9 μm/(m∙K)
Thermal conductivity: 16.4 W/(m∙K)
Electrical Properties of Lutetium
Electrical conductivity: 1.8×106 S/m
sElectrical resistivity: poly: 582 nΩ∙m
sElectrical type: Conductor
Critical Point (Superconducting point): 0.1 K (-273.05 oC, 459.49 oC)
Magnetic Properties of Lutetium
Magnetic type: Paramagnetic
Volume magnetic susceptibility: 0.0000118
Mass magnetic susceptibility: 1.2×10-9 m3/kg
Molar magnetic susceptibility: 2.1×10-10 m3/mol
Physical Property of Lutetium
Density: 9.841 g/cm3 (In solid), 9.3 g/cm3 (In liquid)
Molar volume: 0.00001778 m3/mol
Young’s modulus: 68.6 GPa
Shear modulus: 27.2 GPa
Bulk modulus: 47.6 GPa
Poisson ratio: 0.261
Vickers hardness: 755-1160 MPa
Brinell hardness: 890-1300 MPa
Atomic Property of Lutetium
Oxidation states: 3, 2, 1
Valence Electrons: 5d1 6s2
Ion charge: Lu3+
Ionization energies: 1st: 523.5 kJ/mol 2nd: 1340 kJ/mol 3rd: 2022.3 kJ/mol 4th: 4365.9 5th: 6445.2
Ionic Radius: 84.8 pm (picometer)
Atomic radius: empirical: 174 pm
Covalent radius: 187±8 pm
Filling Orbital: 4f14
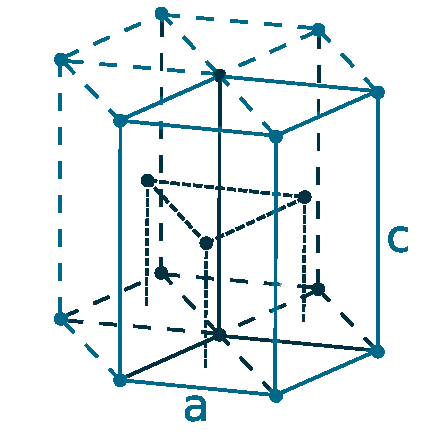
Crystal structure: Hexagonal close-packed
Lattice Angle: π/2, π/2, π/3
Lattice constant: 350.31, 350.31, 555.09 pm
Grid parameters: a=3.503 Å c=5.551 Å
Attitude (c/a): 1.585
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Lutetium
Electronegativity: pauling scale: 1.27
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Lutetium
Half-Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Numbers: 2D3/2
Neutron cross section (Brans): 84
Neutron Mass Absorption: 0.022
Isotopes: 173Lu 174Lu 175Lu 176Lu
| Isotope | Abundance (%) | Atomic Mass (g/mol) | Half-life |
| 173Lu | Syn | – | 1.37 y |
| 174Lu | Syn | – | 3.31 y |
| 175Lu | 97.40 | 174.941 | Stable |
| 176Lu | 2.598 | 175.943 | 3.779×1010 y |
Chemical Reactions
The metal tarnishes slowly in air and burns readily at 150 oC to form Lutetium (lll) oxide:
4 Lu + 3 O2 → 2 Lu2O3
Reacts slowly with cold water and rapidly with hot water (form Lutetium (lll) hydroxide and hydrogen gas):
2 Lu (s) + 6 H2O (l) → 2 Lu(OH)3 (aq) + 3 H2 (g)
The metal reacts with all Halogens to form Lutetium (lll) halides:
2 Lu (s) + 3 F2 (g) → 2 LuF3 (s) [white] (Lutetium (lll) fluoride)
2 Lu (s) + 3 Cl2 (g) → 2 LuCl3 (s) [white] (Lutetium (lll) chloride)
2 Lu (s) + 3 Br2 (g) → 2 LuBr3 (s) [white] (Lutetium (lll) bromide)
2 Lu (s) + 3 I2 (g) → 2 LuI3 (s) [brown] (Lutetium (lll) iodide)
Dissolves readily in dilute sulfuric acid to form Solutions containing Lutetium ions (colourless):
2 Lu + 3 H2SO4 → 2 Lu3+ + 3 SO42– + 3 H2↑
Lutetium metal obtained by reduction of anhydrous Lutetium chloride (LuCl3) or fluoride (LuF3) by either alkali metal or alkaline earth: metal.
2 LuCl3 + 3 Ca → 2 Lu + 3 CaCl2
History of Lutetium
Naming: From Lutetia (the ancient name of Paris, in Roman era)
Discovery: Carl Auer von Welsbach and Georges Urbain (1906)
First isolation: Carl Auer von Welsbach (1906)
Named by: Georges Urbain (1906)
Uses of Lutetium
It’s primary use is in chemical research.
Few commercial uses outside research is as a catalyst for cracking hydrocarbons in oil refineries.
Biological role: It has low toxicity, but it and its compounds should be handled with care.
Abundance of Lutetium
It is a rare earth metal and may be the most expensive of all rare elements.
Main source of lutetium is the ores Monazite and Bastnasite, Where Monazite is also contains Thorium and Ytterbium.
It is extracted, with difficulty, by reducing the anhydrous fluoride with calcium metal.
world wide production is around 10 tons per year.
1×10-8% (In Universe)
1×10-7% (In Sun)
2.9×10-6% (In Meteorites)
0.000057% (In Earth crust)
1.5×10-11% (In Oceans)
World’s Top 3 Producers:
World’s Top 3 Producers of Lutetium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Lutetium
1) China
2) CIS Countries (inc. Russia)
3) USA
#lutetium


