70 Yb (Ytterbium)

Ytterbium is soft, malleable and quite ductile element with an appearance of bright silvery luster.
It should be kept in closed containers to prevent it from air and moisture.
It is easily attacked and dissolved by dilute and concentrated mineral acids and slowly reacts with water.


Identity
CAS Number: 7440-64-4
CID Number: CID23992
RTECS Number: RTECSZG1925000
CONTENT INDEX
Basic Properties of Ytterbium
Appearance: Silvery white; with a pale yellow tint
Mass Number: 173
Standard Atomic weight: 173.045 g/mol
Electrons: 70
Protons: 70
Neutrons: 103
Atomic number (Z): 70
Period: 6
Block: f
Element category: Lanthanide
Electrons per shell: K2, L8, M18, N32, O8, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f146s2

Thermal Properties of Ytterbium
Phase: Solid
Melting point: 1097 K (824 oC, 1515 oF)
Boiling point: 1469 K (1196 oC, 2185 oF)
Fusion heat: 7.66 kJ/mol
Vaporization heat: 129 kJ/mol
Molar heat capacity: 26.74 J/(mol.K)
Thermal expansion: β, poly: 26.3 μm/(m∙K)
Thermal conductivity: 38.5 W/(m∙K)
Electrical properties of Ytterbium
Electrical conductivity: 3.6×106
sElectrical resistivity: β, poly: 0.250 μΩ∙m
sElectrical type: Conductor
Magnetic Properties of Ytterbium
sMagnetic type: Paramagnetic (At 1.0 K)
Magnetic susceptibility (xmol): +249×10-6 cm3
Volume magnetic susceptibility: 0.0000388
Mass magnetic susceptibility: 5.9×10-9 m3/kg
Molar magnetic susceptibility: 1.02×10-9 m3/mol
Physical Properties of Ytterbium
Density: 6.90 g/cm3 (In solid) 6.21 g/cm3 (In Liquid)
Molar volume: 0.00002634 m3/mol
Young’s modulus: β form: 24 GPa
Shear modulus: β form: 9.9 GPa
Bulk modulus: β form: 31 GPa
Poisson ratio: β form: 0.207
Vicker hardness: 205-250 MPa
Brinell hardness: 340-440 MPa
Sound speed: 1590 m/s
Atomic Properties of Ytterbium
Oxidation states: 3,2,1
Valence Electrons: 4f14 6s2
Ion charge: Yb3+ Yb2+
Ionization energies: 1st: 603.4 kJ.mol 2nd: 1174.8 kJ/mol 3rd: 2417 kJ/mol 4th: 4202.9 kJ/mol
Ionic radius: 85.8 pm
Atomic radius: 242 pm (Van der Waals)
Covalent radius: 187±8 pm
Filling Orbital: 4f14
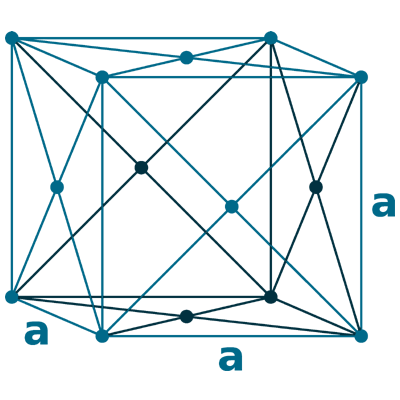
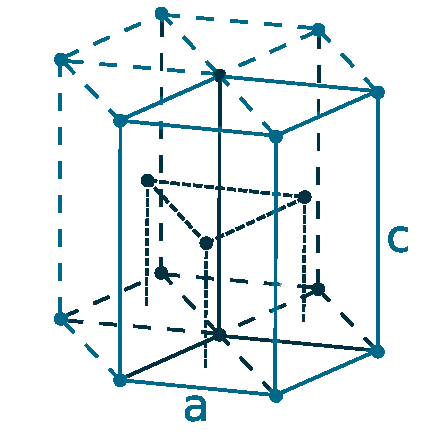
Crystal structure: Face-centered cubic (β, At room temperature), Body centered cubic (γ, At 795 oC), Hexagonal close packed (α, At -13 oC)
Lattice angles: π/2, π/2, π/2
Lattice constant: 548.47, 548.47, 548.47 pm
Grid parameters: 5.490 Å
Space Group Name: Fm_3m
Space Group Number: 225

Reactivity of Ytterbium
Electronegativity: pauling scale: 1.1
Valence: 3
Electron affinity: 50 kJ/mol
Nuclear Properties of Ytterbium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 35
Neutron Mass Absorption: 0.0076
Isotopes: 166Yb 168Yb 169Yb 170Yb 171Yb 172Yb 173Yb 174Yb 175Yb 176Yb 177Yb
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 166Yb | Syn | – | 56.7 h |
| 168Yb | 0.13 | 167.934 | Stable |
| 169Yb | Syn | – | 32.026 d |
| 170Yb | 3.023 | 169.935 | Stable |
| 171Yb | 14.22 | 170.936 | Stable |
| 172Yb | 21.75 | 171.936 | Stable |
| 173Yb | 16.10 | 172.938 | Stable |
| 174Yb | 31.90 | 173.938 | Stable |
| 175Yb | Syn | – | 4.185 d |
| 176Yb | 12.89 | 175.943 | Stable |
| 177yb | Syn | – | 1.911 h |
Chemical Reactions
The metal tarnishes slowly in air and burns readily to form ytterbium (lll) oxide:
4 Yb + 3 O2 → 2 Yb2O3
Reacts slowly with cold water and rapidly with hot water (form Ytterbium (lll) hydroxide and hydrogen gas):
2 Yb (s) + 6 H2O (l) → 2 Yb(OH)3 (aq) + 3 H2 (g)
The metal reacts with all Halogens to form Ytterbium (lll) halides:
2 Yb (s) + 3 F2 (g) → 2 YbF3 (s) [white] (Ytterbium (lll) fluoride)
2 Yb (s) + 3 Cl2 (g) → 2 YbCl3 (s) [white] (Ytterbium (lll) chloride)
2 Yb (s) + 3 Br2 (g) → 2 YbBr3 (s) [white] (Ytterbium (lll) bromide)
2 Yb (s) + 3 I2 (g) → 2 YbI3 (s) [white] (Ytterbium (lll) iodide)
Dissolves readily in dilute sulfuric acid to form Solutions containing Ytterbium (lll) ions (colourless):
2 Yb (s) + 3 H2SO4 (aq) + 18 H2O (l) → 2 [Yb(H2O)9]3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Ytterbium History
Naming: After Ytterby (Village in Sweden), where it was mined
Discovery: Jean Charles Galissard de Marignac (1878)
First isolation: Carl Auer von Welsbach (1906)
Ytterbium Uses
Ytterbium metal could be possible use in improving the grain refinement, strength, and other mechanical properties of stainless steel.
One isotope is have been used as a radiation source substitute for a portable X-ray machine where electricity is unavailable.
Use in memory devices and tuneable lasers.
It can also used as an industrial catalyst and because of low toxicity it is increasingly used to replace other catalysts which considered to be too toxic and polluting.
Some ytterbium alloys have been used in dentistry.
Biological role: It has Low toxicity
Abundance of Ytterbium
Ytterbium is found with other rare elements in several rare minerals such as monazite, Yttria, gadolinite, and xenotime.
It is most often recovered commercially from monazite sand (~0.03% ytterbium).
It can be extracted by ion exchange and solvent extraction.
Annual world wide production is around 50 tons per year.
2×10-7% (In Universe)
1×10-7% (In Sun)
1.8×10-5% (In Meteorites)
0.00028% (In Earth crust)
8×10-11% (In Oceans)
World’s Top 3 producers
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders
1) China
2) CIS Countries (inc. Russia)
3) USA
#ytterbium


