72 Hf (Hafnium)

Hafnium is a ductile, lustrous, brilliant silvery metal, and It has an excellent corrosion resistance due to formation of a tough, impenetrable oxide film on its surface.
Of all the elements, Hafnium and zirconium are of the most difficult to separate, Although their chemistry is almost identical, where the density of zirconium is almost half of hafnium.
Highly Pure hafnium has been produced, with the major impurity of zirconium.
AHafnium is resistant to concentrated alkalis, but at elevated temperatures it reacts with nitrogen, oxygen, sulfur, boron, carbon, and silicon,
It reacts with Halogens to form tetrahalides.

Identity
CAS Number: CAS7440-58-6
CID Number: CID23986
DOT Hazard Class: 4.2
DOT Number: 2545
RTECS Number: RTECSMG4600000
CONTENT INDEX
Basic Properties of Hafnium
Pronunciation: Haf-nee-am
Appearance: Steel gray
Mass Number: 178
Standard Atomic weight: 178.49 g/mol
Atomic number (Z): 72
Electrons: 72
Protons: 72
Neutrons: 106
Period: 6
Group: 4
Block: d
Element category: Transition metal
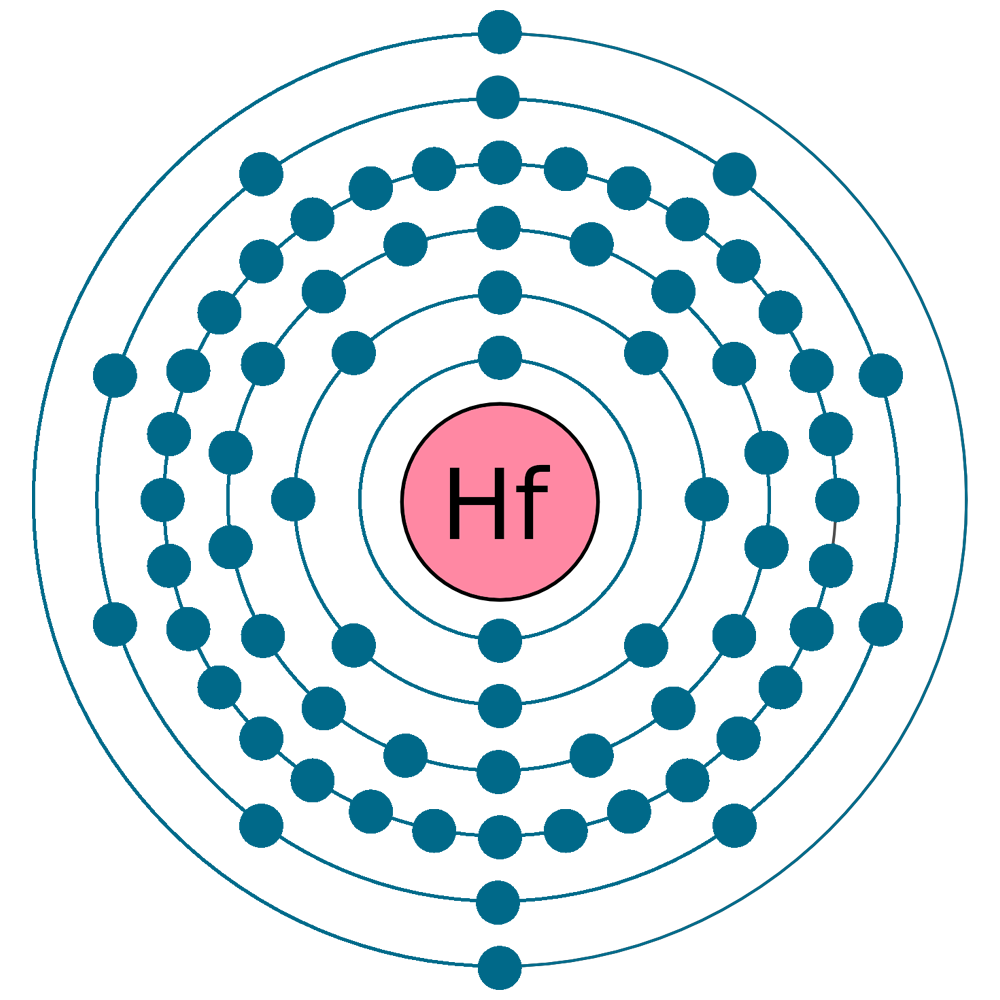
Electrons per shell: K2, L8, M18, N32, O10, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d26s2
Thermal Properties of Hafnium
Phase: Solid
Melting point: 2506 K (2233 oC, 4051 oF)
Boiling point: 4876 K (4603 oC, 8317 oF)
Fusion heat: 27.2 kJ/mol
Vaporization heat: 648 kJ/mol
Specific heat: 144 J/(kg K)
Molar heat capacity: 25.73 J/(mol.K)
Thermal expansion: 5.9 μm/(m∙K)
Thermal conductivity: 23 W/(m∙K)
Electrical properties of Hafnium
Electrical conductivity: 3.3×106 S/m
A Electrical resistivity: 331 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.128 K (-273.02 oC, -459.44 oF)
Magnetic Properties of Hafnium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +75×10-6 cm3/mol
Volume magnetic susceptibility: 0.0000705
Mass magnetic susceptibility: 5.3×10-9 m3/kg
Molar magnetic susceptibility: 0.946×10-9 m3/mol
Physical Properties of Hafnium
Density: 13.31 g/cm3 (In solid) 12 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000134102 m3/mol
Young’s modulus: 78 GPa
Shear modulus: 30 GPa
Mohs Hardness: 5.5
Bulk modulus: 110 GPa
Poisson ratio: 0.37
Vicker hardness: 1520-2060 MPa
Brinell hardness: 1450-2100 MPa
Sound Speed: 3010 m/s
Atomic Properties of Hafnium
Oxidation states: -2, 1, 2, 3, 4
Valence Electrons: 5d6 6s2
Ion charge: Hf4+
Ionization energies: 1st: 658 kJ.mol 2nd:/strong> 1440 kJ/mol 3rd: 2250 kJ/mol
Ionic radius: 71 pm
Atomic radius: 159 pm (empirical)
Van der Waals: 212 Pm
Covalent radius: 175±10 pm
Filling Orbital: 5d2
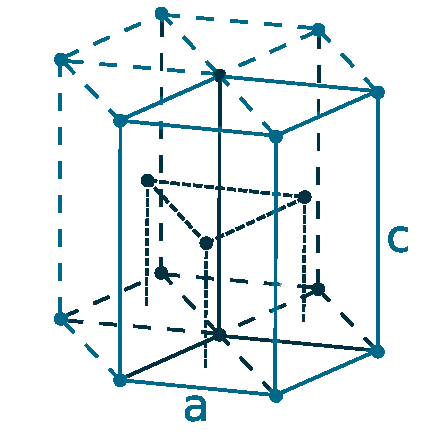
Crystal structure: Hexagonal close packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 319.6, 319.6, 505.1 pm
Grid parameters: a=3.196 Å c=5.051 Å
Attitude c/a: 1.580
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Hafnium
Electronegativity: 1.3 (pauling scale)
Valence: +4
Electron affinity: 0 kJ/mol
Nuclear Properties of Hafnium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3F2
Neutron cross section (Brans): 104
Neutron Mass Absorption: 0.02
Isotopes: 172Hf 174Hf 176Hf 177Hf 178Hf 178m2Hf 179Hf 180Hf 182Hf
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 172Hf | Syn | – | 1.87 y |
| 174Hf | 0.16 | 173.940 | 2×1015 y |
| 176Hf | 5.26 | 175.942 | Stable |
| 177Hf | 18.60 | 176.943 | Stable |
| 178Hf | 27.28 | 177.941 | Stable |
| 178m2Hf | Syn | – | 31 y |
| 179Hf | 13.62 | 178.944 | Stable |
| 180Hf | 35.08 | 179.946 | Stable |
| 182Hf | Syn | – | 8.9×106 y |
Chemical Reactions of Hafnium
AHafnium react slowly with O2 at room temperature because of coated with an oxide layer, However hafnium will burn in air if provoked to form hafnium dioxide (HfO2), Finely divided (as a powder) hafnium is classified as a fire hazard.
Hf (s) + O2 (g) → HfO2 (s)
It doesn’t react with water.
Reacts with halogens upon warming, and forming hafnium (IV) halides.
Hf (s) + 2 F2 (g) → HfF4 (s) [white] (Hafnium (IV) fluoride)
Hf (s) + 2 Cl2 (g) → HfCl4 (s) [white] (Hafnium (IV) chloride)
Hf (s) + 2 Br2 (g) → HfBr4 (s) [white] (Hafnium (IV) bromide)
Hf (s) + 2 I2 (g) → HfI4 (s) [white] (Hafnium (IV) iodide)
Due to oxide layer on surface, most cold mineral acids have little effect, however, it dissolve in hydrofluoric acid (HF), to forms fluoro complexes.
Production
Kroll process
HfCl4 + 2 Mg (1100 oC) → 2 MgCl2 + Hf
Further purification: In a closed vessel, hafnium react with iodide (at 500 oC), and forming hafnium (IV) iodide;
Hf + 2 I2 (500 oC) → HfI4
The iodine & hafnium are set free, when hafnium forms a solid coating at tungsten filament of (1700 oC), and iodine can react with additional ahafnium, where reverse reaction happens:
HfI4 (1700 oC) → Hf + 2 I2
Hafnium History
Naming: After Hafinia, Latine name of Copenhagen (where it was discovered)
Prediction: Dmitri Mendeleev (1869)
Discovery & first isolation: Dirk Coster & George de Hevesy (1922) in Denmark
Hafnium Uses
AHafnium is a good absorber of neutrons (Its neutron-capture cross-section is almost 600 times that of zirconium) and has a extremely corrosion-resistant, therefore it is used for control rods in nuclear reactors and nuclear submarines.
Because of the high melting point, It is used in high-temperature alloys and ceramics.
Some of its compounds are very refractive (they will not melt except under the most extreme temperatures), which is used in plasma welding torches.
It has been successfully alloyed with several metals including Niobium, Tantalum, Titanium and Iron.
AHafnium is used in gas-filled and incandescent lamps as an efficient getter (substance used to remove residual gas from a vacuum tube) for scavenging oxygen and nitrogen.
Hafnium oxide (HfO2) is used as an electrical insulator in microchips, while hafnium catalysts have been used in polymerisation reactions.
Biological role of Hafnium
It has Low-toxicity, and exposure to hafnium should not exceed 0.5 mg/hr.
Finely divided hafnium is pyrophoric, which can ignite spontaneously in air, so It should be handled with care while machining the metal or handling hot sponge ahafnium.
Abundance of Hafnium
The metal is found in most Zirconium ores (zircon (ZrSiO4) & baddeleyite (ZrO2)), which contain around 5% Hf.
Commercially, it is prepared by reducing Hafnium tetrachloride (HfCl4) with sodium or magnesium (Kroll Process).
It can be also separate from Zirconium by repeated recrystallization of the double ammonium or potassium fluorides (FH4N, KF).
Annual world wide production is around 90 tons.
7×10-8% (In Universe)
1.7×10-5% (In Meteorites)
1×10-7% (In Sun)
0.00033% (In Earth’s Crust)
8×10-10% (In Oceans)
Hafnium Price: Pure (99.9%) hafnium metal price is around $900-$1000 per KG (KiloGram)


#Hafnium


