53 I (Iodine)

Iodine is a non-metallic, dark-gray or bluish-black, lustrous solid element.
It forms compounds with many elements, but least reactive than the other halogens.
Iodine sublime (change directly into vapour when heated) easily on heating to give a purple colour.
Iodine dissolves readily in some solvent, such as carbon tetrachloride (CCl4), chloroform (CHCl3), or carbon disulfide (CS2) to form beautiful purple solutions, and slightly soluble in water.


Identity
CAS Number: CAS7553-56-2
CID Number: CID807
DOT Hazard Class: 8
DOT Number: 1759
RTECS Number: RTECSNN1575000
CONTENT INDEX
Basic Properties of Iodine
Pronunciation: Eye-o-deen
Appearance: Lustrous metallic gray (violet as a gas)
Mass Number: 127
Standard Atomic weight: 126.904 g/mol
Atomic number (Z): 53
Electrons: 53
Protons: 53
Neutrons: 74
Period: 5
Group: 17
Block: p
Element category: Halogens
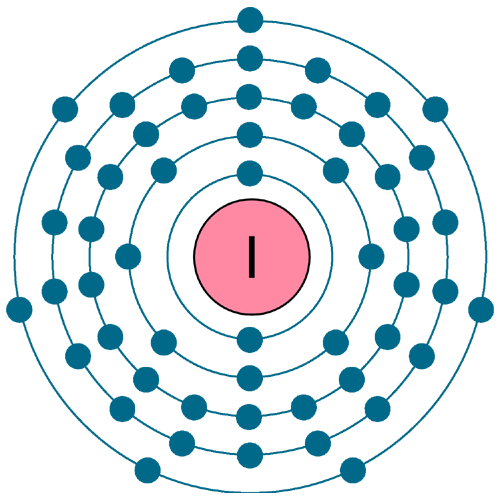
Electrons per shell: K2, L8, M18, N18, O7
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p5
Thermal Properties of Iodine
Phase: Solid
Melting point: 386.84 K (113.7 oC, 236.65 oF)
Boiling point: 457.3 K (184.3 oC, 363.6 oF)
Critical temperature: 819 K (545.85 oC, 1014.53 oF)
Critical Pressure: 11.7 MPa (115.47 Atm)
Triple point temperature: 386.65 K (113.5 oC, 236.3 oF)
Triple point Pressure: 12.1 kPa (0.119 Atm)
Fusion heat: 15.52 kJ/mol
Vaporization heat: 41.57 kJ/mol
Molar heat capacity: 54.44 J/(mol.K)
Thermal conductivity: 0.449 W/(m∙K)
Electrical properties of Iodine
Electrical conductivity: 1×106 S/m
A Electrical resistivity: 1.3×107 Ω∙m
A Electrical type: Insulator
Magnetic Properties of Iodine
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -88.7×10-6 cm3/mol
Volume magnetic susceptibility: – 0.0000222
Mass magnetic susceptibility: -4.5×10-9 m3/kg
Molar magnetic susceptibility: -0.571×10-9 m3/mol
Physical Properties of Iodine
Density: 4.933 g/cm3
Molar volume: 0.00002569 m3/mol
Bulk modulus: 7.7 GPa
Atomic Properties of Iodine
Oxidation states: 7, 6, 5, 4, 3, 1, -1
Valence Electrons: 5s2 5p5
Ion charge: I–
Ionization potential of an atom: 10.4
Ionization energies: 1st: 1008.4 kJ.mol 2nd: 1845.9 kJ/mol 3rd: 3180 kJ/mol
Ionic radius: 220 pm
Atomic radius: 198 pm (Van der Waals)
Covalent radius: 139±3 pm
Filling Orbital: 5p5
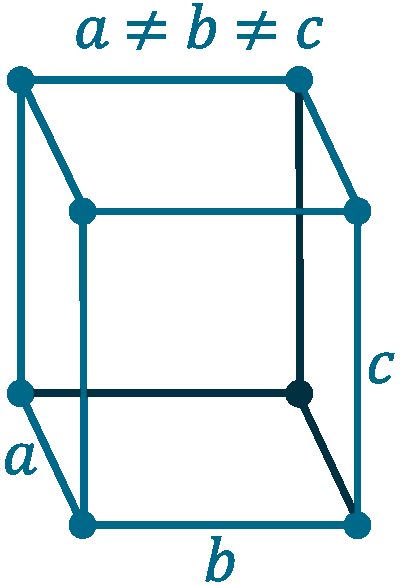
Crystal structure: Orthorhombic
Lattice angles: π/2, π/2, π/2
Lattice constant: 718.02, 471.02, 981.03 pm
Grid parameters: a=7.18 Å b=4.71 Å c=9.81 Å
Space Group Name: Cmca
Space Group Number: 64
Reactivity of Iodine
Electronegativity: pauling scale: 2.66
Valence: +7
Electron affinity: 295.2 kJ/mol
Nuclear Properties of Iodine
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2P3/2
Neutron cross section (Brans): 6.2
Neutron Mass Absorption: 0.0018
Isotopes: 123I 124I 125I 127I 129I 131I 135I
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 123I | Syn | – | 13 h |
| 124I | Syn | – | 4.176 d |
| 125I | Syn | – | 59.40 d |
| 127I | 100 | 126.904 | Stable |
| 129I | Trace | – | 1.57×107 y |
| 131I | Syn | – | 8.02070 d |
| 135I | Syn | – | 6.57 h |
Chemical Reactions
Iodine (I2) does not react with air (oxygen O2 or Nitrogen N2). However, it reacts with ozone (O3) and forming the unstable dark yellow Tetraiodine nonoxide (I4O9)
Iodine reacts with water and Forming Hypoiodite IO– :
I2 (aq) + H2O (l) ⇌ IO– + 2 H+ (aq) + I– (aq)
It reacts with other Halogens:
I2 (s) + 5 F2 (g) → 2 IF5 (l) [colourless] (Pentafluoride iodine (V) fluoride, At room temp.)
I2 (g) + 7 F2 (g) → 2 IF7 (g) [colourless] (heptafluoride iodine (VII) fluoride, At 250 oC)
I2 (s) + 3 F2 (g) → 2 IF3 (s) [yellow] (trifluoride iodine (III) fluoride, At -45 oC)
I2 (s) + Br2 (l) → 2 IBr (s) (Iodine (I) bromide)
I2 (s) + 3 Cl2 (l) → I2Cl6 (s) [yellow] (iodine tri-chloride)
Reacts with chlorine in the presence of water to form iodic acid.
I2 (s) + 6 H2O (l) + 5 Cl2 (g) → 2 HIO3 (s) + 10 HCl (g)
Reacts with hydrogen and forming hydrogen iodide:
H2 (g) + Br2 (g) → 2 HBr (g) (reaction speed increase with increase in temperature)
I2 Reacts with concentrated nitric acid and forming iodic acid
I2 (s) + 10 HNO3 (aq) → 2 HIO3 (s) + 10 NO2 (g) + 4 H2O (g)
Reacts with (bases) hot aqueous alkali and forming iodate IO3–
3 I2 (s) + 6 OH– (aq) → IO3– (aq) + 5 I– (aq) + 3 H2O (l)
Iodine History
Naming: Greek: iôdes (violet)
Discovery and first isolation: Bernard Courtois (1811)
Iodine Uses
Iodine compounds are important in organic chemistry and useful in medical treatment as iodine tincture (It is a solution of KI and iodine in alcohol is used as antiseptic for external wounds) and iodoform.
Other uses are in printing inks and dyes, catalysts, animal feed supplements and photographic chemicals (Potassium iodide (KI) is used in photography).
It is also used to make polarizing filters for LCD displays.
The radioactive isotope 131I has been used to treat cancerous thyroid glands
Biological role of Iodine
Iodine is very essential for humans, daily intake need of about 0.1 milligrams of iodide.
Human body contains iodine up to 20 milligrams, mainly in thyroid gland. This gland helps to regulate growth, the nervous system and the metabolism.
Normally we get enough iodine from the food we eat.
Due to shortage of iodine in human body, the function of the thyroid gland will slow down and that gland will start swelling up. This phenomenon is called struma.
Large quantity of iodine can be dangerous for human body, because the thyroid gland will work too hastily that affects the entire body, It causes loss of weight and disturbed heartbeats.
I2 is toxic, and its vapour irritates the eyes and lungs, even all iodides are toxic if it taken in excess.
Iodine 131 is a radionuclides (radioactive isotope) that increases the risk of cancer and possibly other diseases caused by thyroid hormonal deficiency.
Abundance of Iodine
Iodine is found in seawater in form of iodide, present 0.05 parts per million. Iodine obtained from seaweeds in the past.
Now a days, The main sources of iodine are iodate minerals, such as alutarite (found in chile) and iodargyrite (found in Colorado, naveda and new maxico)
It is obtained by releasing iodine from iodate which is obtained from nitrate ores or extracting iodine vapour from the processed brine.
Ultrapure iodine can be obtained from the reaction of potassium iodide (KI) with copper sulfate (CuSO4).
Annual world wide production of iodine is around 12,000 tons.
1×10-7% (In Universe)
2.5×10-5% (In Meteorites)
0.000049% (In Earth’s Crust)
6×10-6% (In Oceans)
0.00002% (In Humans)
World’s Top 3 producers of Iodine
1) Chile
2) Japan
3) USA
World’s Top 3 Reserve holders of Iodine
1) Chile
2) Japan
3) USA
#iodine


