24 Cr (Chromium Element)

Chromium is a silver-gray, lustrous, hard and brittle metal. Which can be highly polished and does not tarnish in air. But when heated, It forms the green chromic oxide.
It is unstable in oxygen, it immediately produces a thin oxide layer that is impermeable (not allowing fluid to pass through) to oxygen and protects the metal below.

Identity
CAS Number: CAS7440-47-3
CID Number: CID23976
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSGB4200000
CONTENT INDEX
Basic Properties of Chromium
Appearance: Silvery Metallic
Mass Number: 52
Standard Atomic weight: 51.996 g/mol
Atomic number (Z): 24
Electrons: 24
Protons: 24
Neutrons: 28
Period: 4
Group: 6
Block: d
Element category: Transition metal
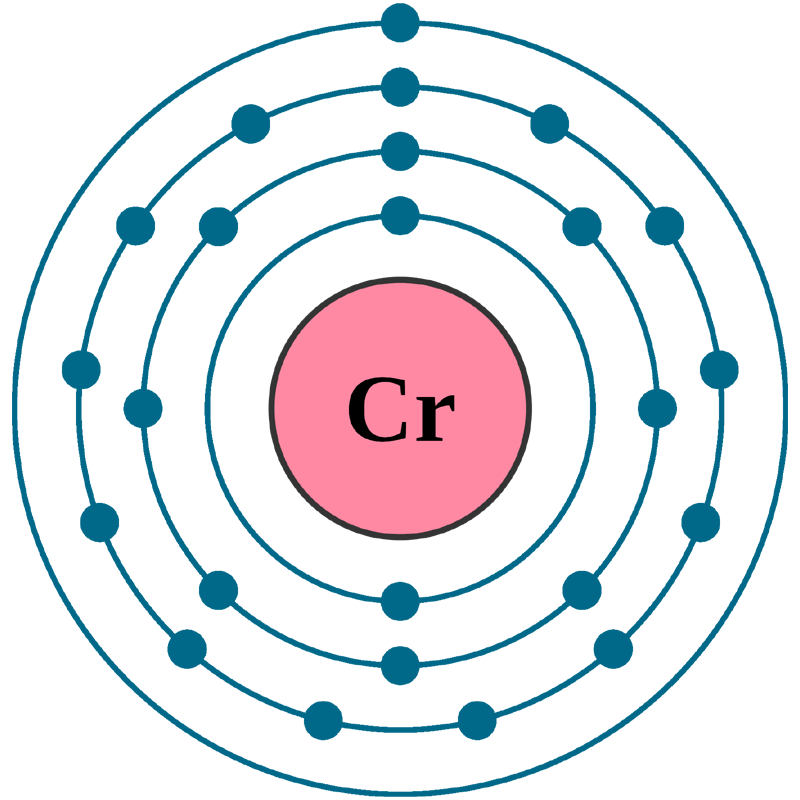
Electrons per shell: K2, L8, M13, N1
Electron configuration: 1s22s22p63s23p63d54s1
Thermal Properties of Chromium
Phase: Solid
Melting point: 2180 K (1907 oC, 3465 oF)
Boiling point: 2944 K (2671 oC, 4840 oF)
Debye temperature: 460 K (186.85 oC, 368.33 oF)
Fusion heat: 21.0 kJ/mol
Vaporization heat: 347 kJ/mol
Specific heat: 448 J/(kg K)
Molar heat capacity: 23.34 J/(mol.K)
Thermal expansion: 4.9 μm/(m∙K)
Thermal conductivity: 93.9 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 393 K (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Chromium
Electrical conductivity: 7.9×106 S/m
A Electrical resistivity: 125 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Chromium
A Magnetic type: Antiferromagnetic
Magnetic susceptibility (xmol): +280×10-6 cm3/mol
Volume magnetic susceptibility: 0.0003177
Mass magnetic susceptibility: 44.5×10-9 m3/kg
Molar magnetic susceptibility: 2.314×10-9 m3/mol
Physical Properties of Chromium
Density: 7.19 g/cm3 (In solid) 6.3 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000072317 m3/mol
Young’s modulus: 278 GPa
Shear modulus: 115 GPa
Mohs Hardness: 8.5
Bulk modulus: 160 GPa
Poisson ratio: 0.21
Vicker hardness: 1060 MPa
Brinell hardness: 687-6500 MPa
Sound Speed: 5940 m/s
Atomic Properties of Chromium
Oxidation states: -4, -2, -1, 1, 2, 3, 4, 5, 6
Valence Electrons: 3d5 4s1
Ion charge: Cr3+ Cr2+
Ionization potential of an atom: 6.74
Ionization energies: 1st: 653.89 kJ.mol 2nd: 1590.59 kJ/mol 3rd: 2987 kJ/mol
Ionic radius: 52 pm
Atomic radius: 128 pm (empirical)
Van der Waals: 189 Pm
Covalent radius: 139±5 pm
Filling Orbital: 3d5
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 288.5, 288.5, 288.5 pm
Grid parameters: a=2.885 Å
Space Group Name: Im_3m
Space Group Number: 229

Reactivity of Chromium
Electronegativity: 1.66 (pauling scale)
Valence: +6
Electron affinity: 64.3 kJ/mol
Nuclear Properties of Chromium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 7S3
Neutron cross section (Brans): 3.1
Neutron Mass Absorption: 0.0021
Isotopes: 50Cr 51Cr 52Cr 53Cr 54Cr
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 50Cr | 4.345 | 49.946 | Stable |
| 51Cr | Syn | – | 27.70 d |
| 52Cr | 83.789 | 51.941 | Stable |
| 53Cr | 9.501 | 52.941 | Stable |
| 54Cr | 2.365 | 53.939 | Stable |
Chemical Reactions of Chromium
AChromium doesn’t react with air and water, at normal temperature.
Chromium reacts directly with fluorine (F2), at 400°C and 200-300 atmospheric pressure, it forms:
Cr (s) + 3 F2 (g) → CrF6 (s) [yellow] (chromium (VI) fluoride)
Under milder conditions, it formed:
2 Cr (s) + 5 F2 (g) → 2 CrF5 (s) [red] (chromium(V) fluoride)
Under still milder conditions, The metal reacts with the halogens, and forms Chromium trihalides:
2 Cr (s) + 3 F2 (g) → 2 CrF3 (s) [green] (chromium (III) fluoride)
2 Cr (s) + 3 Cl2 (g) → 2 CrCl3 (s) [red-violet] (chromium (III) chloride)
2 Cr (s) + 3 Br2 (g) → 2 CrBr3 (s) [very dark green] (chromium (III) Bromide)
2 Cr (s) + 3 I2 (g) → 2 CrI3 (s) [very dark green] (chromium (III) Iodide)
Metallic chromium dissolves in dilute hydrochloric acid (HCL), and forming Cr(II) and hydrogen gas (H2), and in this aqueous solution, Cr (II) is present as the complex ion [Cr(OH2)6]2+.
Cr (s) + 2 HCL (aq) → Cr2+ (aq) +2 Cl– (aq) + H2 (g)
Similar results are seen for sulphuric acid (H2SO4) but pure samples of chromium may be resistant to attack.
Chromium metal does not react with nitric acid (HNO3) and in fact is passivated (make unreactive by altering the surface layer or coating the surface with a thin inert layer).
Reaction of Chromate anion (CrO42-) and Dichromate (Cr2O72-) anions exist in equilibrium:
2 [CrO4]2- + 2H+ ⇌ [Cr2O7]2- + H2O
A Reaction shows Both the chromate and dichromate anions are strong oxidizing reagents at low pH:
Cr2O72- + 14 H3O+ + 6 e– → 2 Cr3+ + 21 H2O [ε0 = 1.33 V]
Reaction of, moderately oxidizing at high pH:
CrO42- + 4 H2O + 3 e– → Cr(OH)3 + 5 OH– [ε0 = -0.13 V] –
Chromium History
Naming: Nicholas Louis Vauquelin
Discovery and first isolation: Louis Nicolas Vauquelin (1794, 1797) In Paris (France)
Chromium Uses
TheChromium is used to harden steel, to manufacture stainless steel (Contains10.5% Chromium) and to produce several useful alloys.
AChromium is mostly used in plating to give a steel a polished silvery mirror coating (corrosion resistance), also to produce a hard, beautiful surface, Which is commonly used in Chromium-plated car and lorry parts, such as bumpers.
All compounds of chromium are colored, and The most important chromates are those of sodium and potassium dichromate (Na2Cr2O7 & K2Cr2O7) , and the potassium and ammonium chrome alums (Chromium (III) potassium sulfate, KCr(SO4)2).
The dichromates are used as oxidizing agents in quantitative analysis, to make molds for the firing of bricks, and also used in tanning leather (the waste effluent is toxic so alternatives are being investigated).
AChromium compounds are widely used as industrial catalysts and pigments (in bright green, red yellow, and orange colours).
Rubies get their red colour, and glass gets emerald green color from chromium.
CrO2 (Chromium (IV) oxide) is used to manufacture magnetic tape.
Chromium compounds are also used in the textile industry as mordants, and by the aircraft and other industries for anodizing aluminum (by electrochemical process).
Biological role of Chromium
Chromium is an essential trace element for humans because it helps us to use glucose (in the action of insulin), We daily intake about 1 mg (milligram). However, it will be poisonous in excess quantity.
Foods such as brewer’s yeast (Saccharomyces cerevisiae), wheat germ and kidney are rich in chromium.
Chromium compounds are toxic and should be Proper handled with care.
Hexavalent chromium (Cr(VI) or Cr6+) is highly toxic and mutagenic (a physical or chemical agent that changes the genetic material, usually DNA) when inhaled
Abundance of Chromium
Chromium is found mainly in chromite (FeCr2O4) ore.
Commercially, The metal is usually produced by reducing chromium(III) oxide with aluminum or silicon, or by reducing chromite with carbon in an electric-arc furnace.
Annual world wide production is around 8,00,000 tons.
0.0015% (In Universe)
0.3% (In Meteorites)
0.002% (In Sun)
0.014% (In Earth’s Crust)
6×10-8% (In Oceans)
3×10-6% (In Humans)

World’s Top 3 producers of Chromium
1) South Africa
2) Kazakhstan
3) India
World’s Top 3 Reserve holders of Chromium
1) Kazakhstan
2) South Africa
3) India
#Chromium


