52 Te (Tellurium)

Tellurium is a semimetallic, crystalline, silver-white element. It exhibits metallic luster in pure form.
It is brittle, so it can easily pulverized.
A It forms many compounds corresponding to those of selenium and sulfur.
A Tellurium burned in air with a greenish-blue flame and forms tellurium dioxide (TeO2),
Amorphous tellurium is found by precipitating tellurium from a solution of telluric or tellurous acid (H2TeO3).
Tellurium is a p-type semiconductor, and its conductivity increases slightly with exposure to light.


Identity
CAS Number: CAS13494-80-9
CID Number: CID6327182
DOT Hazard Class: 6.1
DOT Number: 2811
RTECS Number: RTECSWY2625000
CONTENT INDEX
Basic Properties of Tellurium
Pronunciation: Ta-lewr-ee-am
Appearance: Silver lustrous gray (crystalline) & Brown-black powder (amorphous)
Mass Number: 128
Standard Atomic weight: 127.60 g/mol
Atomic number (Z): 52
Electrons: 52
Protons: 52
Neutrons: 76
Period: 5
Group: 16
Block: p
Element category: Metalloid (semi metal)
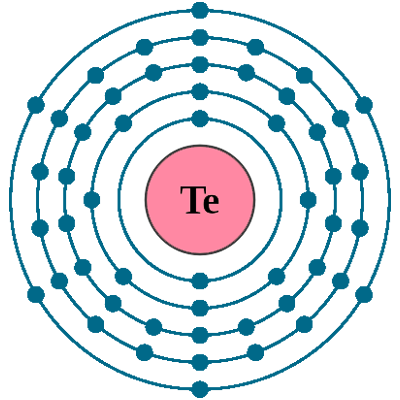
Electrons per shell: K2, L8, M18, N18, O6
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p4
Thermal Properties of Tellurium
Phase: Solid
Melting point: 722.66 K (449.51 oC, 841.12 oF)
Boiling point: 1261 K (988 oC, 1810 oF)
Fusion heat: 17.49 kJ/mol
Vaporization heat: 114.1 kJ/mol
Specific heat: 201 J/(kg K)
Molar heat capacity: 25.73 J/(mol.K)
Thermal expansion: 18 μm/(m∙K)
Thermal conductivity: 1.97-3.38 W/(m∙K)
Electrical properties of Tellurium
Electrical conductivity: 0.01×106 S/m
A Electrical resistivity: 0.0001 Ω∙m
A Electrical type: SemiConductor (p-type)
Magnetic Properties of Tellurium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -39.5×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000243
Mass magnetic susceptibility: -3.9×10-9 m3/kg
Molar magnetic susceptibility: -0.498×10-9 m3/mol
Physical Properties of Tellurium
Density: 6.24 g/cm3 (In solid) 5.70 g/cm3 (In Liquid)
Molar volume: 0.00002045 m3/mol
Young’s modulus: 43 GPa
Shear modulus: 16 GPa
Mohs Hardness: 2.25
Bulk modulus: 65 GPa
Brinell hardness: 180-270 MPa
Refractive index: 1.000991
Sound Speed: 2610 m/s
Atomic Properties of Tellurium
Oxidation states: 6, 5, 4¸ 3, 2, 1, -1, -2
Valence Electrons: 5s2 5p4
Ion charge: Te2-
Ionization energies: 1st: 869.3 kJ.mol 2nd: 1790 kJ/mol 3rd: 2698 kJ/mol
Ionic radius: 97 pm
Atomic radius: 206 pm (Van der Waals)
Covalent radius: 138±4 pm
Filling Orbital: 5p4
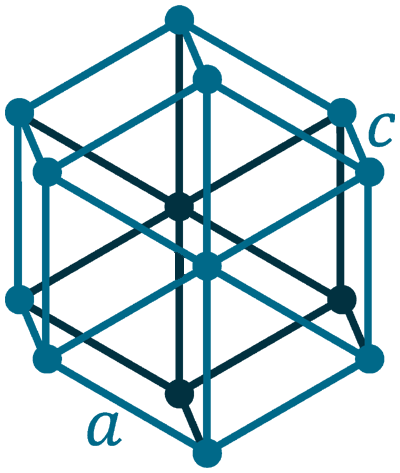
Crystal structure: Hexagonal
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 445.72, 445.72, 592.9 pm
Grid parameters: a=4.457 Å c=5.929 Å
Attitude c/a: 1.330
Space Group Name: P3121
Space Group Number: 152
Reactivity of Tellurium
Electronegativity: pauling scale: 2.1
Valence: +6
Electron affinity: 190.2 kJ/mol
Nuclear Properties of Tellurium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P2
Neutron cross section (Brans): 5.4
Neutron Mass Absorption: 0.0013
Isotopes: 120Te 121Te 122Te 123Te 124Te 125Te 126Te 127Te 128Te 129Te 130Te
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 120Te | 0.09 | 119.904 | Stable |
| 121Te | Syn | – | 16.78 d |
| 122Te | 2.55 | 121.903 | Stable |
| 123Te | 0.89 | 122.904 | Stable |
| 124Te | 4.74 | 123.903 | Stable |
| 125Te | 7.07 | 124.904 | Stable |
| 126Te | 18.84 | 125.903 | Stable |
| 127Te | Syn | – | 9.35 h |
| 128Te | 31.74 | 127.904 | 2.2×1024 y |
| 129Te | Syn | – | 69.6 min |
| 130Te | 34.08 | 129.906 | 7.9×1020 y |
Chemical Reactions
Tellurium is uneffected by water or hydrochloric acid, but dissolves in nitric acid.
The metal burns in air to form tellurium (IV) oxide:
Te (s) + 2 O2 (g) → TeO2 (s)
The metal reacts with Fluorine:
Te8 (s) + 24 F2 (g) → 8 TeF6 (l) (tellurium (VI) fluoride)
Reacts with fluorine in nitrogen atmoshphere at 0 oC)
Te8 (s) + 16 F2 (g) → 8 TeF4 (s) (tellurium (IV) fluoride)
React with other halogens in Nitrogen atmosphere at 0 oC to form tetra halides:
Te8 (s) + 16 Cl2 (g) → 8 TeCl4 (s)
Te8 (s) + 16 Br2 (g) → 8 TeBr4 (s)
Te8 (s) + 24 I2 (g) → 8 TeI4 (s)
Production
The first step usually involves an oxidation in the presence of sodium carbonate (soda ash).
M2Te + O2 + Na2CO3 → Na2TeO3 + 2M + CO2 (M= Cu, Ag, Au)
Put Cu in place of M in above reaction:
Cu2Te + Na2CO3 + O2 → 2 Cu + Na2TeO3 + CO2
OR
Cu2Te + Na2CO3 + 2 O2 → 2 CuO + Na2TeO3 + CO2
The Tellurite (Na2TeO3) is separated by sulphuric acid (H2SO4) and tellurium precipitates out as the dioxide. Tellurium is liberated from the dioxide by dissolving in sodium hydroxide, NaOH, and electroytic reduction.
TeO2 + 2 NaOH → Na2TeO3 + H2O → Te + 2 NaOH + O2
Tellurium History
Naming: After Roman Tellus, deity of the earth
Discovery: Franz-Joseph Muller von Reichenstein (1782)
First isolation: Martin Heinrich Klaproth
Tellurium Uses
Tellurium is used in alloys, mostly with aluminium, tin, copper and stainless steel, to improve their machinability. It is added to lead to improve its strength and hardness and makes corrosive resistant to sulphuric acid on lead.
Tellurium has been used as a basic ingredient in blasting caps, to vulcanize rubber, to tint glass and ceramics, in solar cells, in rewritable CDs and DVDs,as a catalyst in oil refining, and is added to cast iron for chill control.
A Tellurium can be doped with gold, silver, copper or tin in semiconductor applications.
Bismuth telluride has been used in thermoelectric devices.
Biological role: It is very toxic and teratogenic (it is an agent that can disturbs the development of an embryo or foetus).
Abundance of Tellurium
Tellurium is occasionally found in minerals calaverite, sylvanite and tellurite, but more often found in the telluride.
It is obtained commercially from anode sludges produced during the electrolytic refining of blister copper.
Obtain 1 Kg (2.2 Pounds) of tellurium by treatment of 1000 tons of copper ore.
Annual world wide production is around 215 tons.
9×10-7% (In Universe)
21×10-5% (In Meteorites)
1×10-7% (In Earth’s Crust)



World’s Top producers of Tellurium
1) USA
2) Peru
3) Canada
4) Japan
#tellurium


