51 Sb (Antimony Element)

CONTENT INDEX
About Antimony Element
It is exist in two forms,
(1) The metallic form is silvery, bright, hard and brittle
(2) The non metallic form is a grey powder.
Antimony is a poor conductor of heat and electricity.


Identity
CAS Number: CAS7440-36-0
CID Number: CID5354495
RTECS Number: RTECSCC4025000
Basic Properties of Antimony Element
Pronunciation: an-ta-ma-nee
Appearance: Silvery lustrous gray
Alternate Names: Stibium
Names of Allotropes: White Antimony, Yellow Antimony, Black Antimony
Mass Number: 122
Standard Atomic weight: 121.760 g/mol
Atomic number (Z): 51
Electrons: 51
Protons: 51
Neutrons: 71
Period: 5
Group: 15
Block: p
Element category: Metalloid (semi-metal)
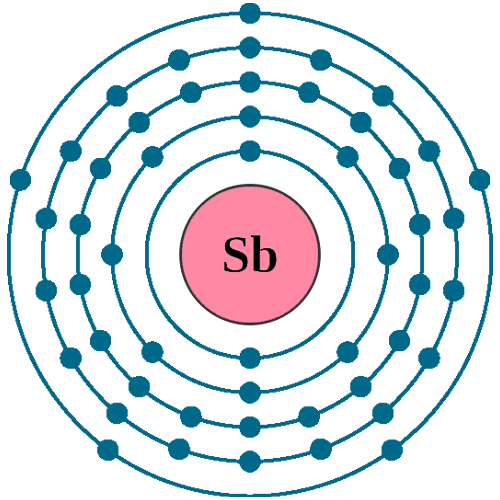
Electrons per shell: K2, L8, M18, N18, O5
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p3
Thermal Properties of Antimony
Phase: Solid
Melting point: 903.78 K (630.63 oC, 1167.13 oF)
Boiling point: 1908 K (1635 oC, 2975 oF)
Debye temperature: -73.15 K (99.67 oC,200 oF)
Fusion heat: 19.79 kJ/mol
Vaporization heat: 193.43 kJ/mol
Specific heat: 207 J/(kg K)
Molar heat capacity: 25.23 J/(mol.K)
Thermal expansion: 11 μm/(m∙K)
Thermal conductivity: 24.4 W/(m∙K)
Electrical properties of Antimony
Electrical conductivity: 2.5×106 S/m
A Electrical resistivity: 417 nΩ∙m
A Electrical type: Conductor (poor conductor)
Magnetic Properties of Antimony
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -99×10-6 cm3/mol
Volume magnetic susceptibility: 0.000073
Mass magnetic susceptibility: 10.9×10-9 m3/kg
Molar magnetic susceptibility: 1.327×10-9 m3/mol
Physical Properties of Antimony
Density: 6.697 g/cm3 (In solid) 6.53 g/cm3 (In Liquid)
Molar volume: 0.00001818 m3/mol
Young’s modulus: 55 GPa
Shear modulus: 20 GPa
Mohs Hardness: 3
Bulk modulus: 42 GPa
Brinell hardness: 294 MPa
Sound Speed: 3420 m/s
Atomic Properties of Antimony
Oxidation states: 5, 4, 3,2, 1, -1, -2, -3
Valence Electrons: 5s2 5p3
Ion charge: Sb3+ Sb5+
The ionization potential of an atom: 8.35
Ionization energies: 1st: 834 kJ.mol 2nd: 1594.9 kJ/mol 3rd: 2440 kJ/mol
Ionic radius: 76 pm
Atomic radius: 206 pm (Van der Waals)
Covalent radius: 139±5 pm
Filling Orbital: 5p3
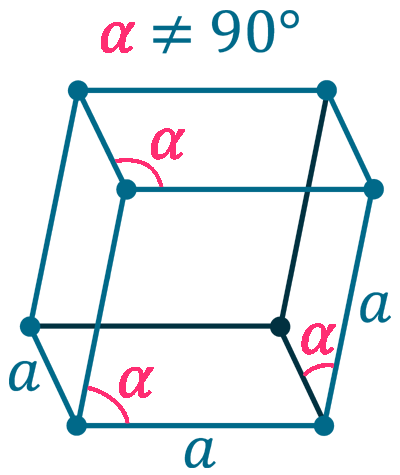
Crystal structure: Rhombohedral
Lattice constant: 430.7, 430.7, 1127.3 pm
Grid parameters: a(Hex)=4.307 Å c(Hex)=11.27 Å
Attitude c/a: 2.62
Space Group Name: R_3m
Space Group Number: 166
Reactivity of Antimony
Electronegativity: pauling scale: 2.05
Valence: +5
Electron affinity: 103.2 kJ/mol
Nuclear Properties of Antimony Element
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4S3/2
Neutron cross section (Brans): 5.4
Neutron Mass Absorption: 0.0016
Isotopes: 121Sb 123Sb 125Sb
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 121Sb | 57.21 | 120.904 | Stable |
| 123Sb | 42.79 | 122.904 | Stable |
| 125Sb | Syn | – | 2.7582 y |
Chemical Reactions
Antimony reacts with in air and form trioxide antimony:
4 Sb (s) + 3 O2 (g) → 2 Sb2O3 (s) (Flame is Bluish white)
At red heat, Reacts with water and form Antimony (lll) oxide: Sb reacts slowly at ambient temperature.
2 Sb (s) + 3 H2O (g) → Sb2O3 (s) + 3 H2 (g)
Sb (III) as the tetra chloro complex can react with water and forming the precipitate Antimony oxychloride (SbOCl):
[SbCl4]– (aq) + H2O (l) ⇌ SbOCl (s) [white] + 3 Cl‑ (aq) + 2 H+ (aq)
The Precipitate can be dissolved by alkali:
SbOCl (s) + 2 OH– (aq) + H2O (l) ⇌ [Sb(OH)4]– (aq) + Cl– (aq)
2 SbOCl (s) + 2 C4H6O6 (aq) ⇌ [Sb2(C4H6O6)2]2- (aq) + 4 H+ (aq) + 2 Cl– (aq) + 2 H2O (l)
Reacts with all Halogens:
2 Sb (s) + 3 F2 (g) → 2 SbF3 (s) [white] (Antimony (lll) fluoride)
2 Sb (s) + 3 Cl2 (g) → 2 SbCl3 (s) [white] (Antimony (lll) chloride)
2 Sb (s) + 3 Br2 (g) → 2 SbBr3 (s) [white] (Antimony (lll) bromide)
2 Sb (s) + 3 I2 (g) → 2 SbI3 (s) [Red] (Antimony (lll) iodide)
Sb React with acid:
Antimony (Sb) dissolved in hot concentrated sulphuric acid (H2SO4) or nitric acid (HNO3) and forming a Sb(III) solutions. The sulphuric acid reaction produces Sulphur dioxide SO2 gas. Antimony does not react with hydrochloric acid (HCl) in the absence of oxygen.
Production
Antimony can be Separated from the crude antimony sulfide by reduction with scrap iron:
Sb2S3 + 3 Fe → 2 Sb + 3 FeS
Antimony is Separated from the oxide by a carbothermal reduction:
2 Sb2O3 + 3 C → 4 Sb + 3 CO2
The lower-grade ores are reduced in blast furnaces while the higher-grade ores are reduced in reverberatory furnaces.
Antimony History
Naming: Greek: anti (not) monos (alone); Symbol for Latin stibium
Discovery: Before 800 CE
Antimony Uses
Pure antimony used in electronics industry to make certain types of semiconductor devices, such as infrared detector, diodes and hall-effect devices.
A Antimony is alloyed with lead to improve their hardness and mechanical strength.
A Antimony alloys are used in Batteries, antifriction alloys (such as Babbitt metal), type metal (linotype printing machines), electrical cable sheathing, small arms and tracer bullets.
Antimony is mainly used in the trioxide for make flame-proofing compounds, paint, glass art, and as an opacifier (Titanium dioxide, TiO2) in enamel.
Biological role: Antimony and many of its compounds are toxic. Inhalation of antimony trioxide is considered harmful and suspected of causing cancer.
Abundance of Antimony
Antimony is not an abundant element, but is found in over 100 mineral species.
Antimony can be found as the native metal.
It is most often found in Stibnite (is a sulfide mineral with the formula, Sb2S3) Sometimes called antimonite.
It is extracted by roasting the antimony trisulfide (Sb2S3) to the oxide, and then reducing with carbon.
Annual world wide production is around 53,000 tons.
4×10-8% (In Universe)
1.2×10-5% (In Meteorites)
1×10-7% (In Sun)
0.000028% (In Earth’s Crust)
2×10-8% (In Oceans)

World’s Top 3 producers of Antimony
1) China (77 %)
2) Russia (7 %)
3) Tajikistan (6 %)
World’s Top 3 Reserve holders of Antimony
1) China
2) Russia
3) Bolivia
#antimony


