40 Zr (Zirconium)

It is a very strong, malleable, ductile, lustrous silver-gray metal.
It is extremely resistant to corrosion by many common acids and alkalis, by sea water, and by other agents.
It is lighter than steel and its hardness is similar to copper.
Zirconium powder is black and is regarded as very dangerous fire hazard, It can spontaneously ignite in air, especially at high temperatures.
Zirconium Alloyed with zinc, becomes magnetic at temperatures below 35°K (-238.15 oC, -398.67 oC).

Identity
CAS Number: CAS7440-67-7
CID Number: CID23995
DOT Hazard Class: 4.2
DOT Number: 2008
CONTENT INDEX
Basic Properties of Zirconium
Pronunciation: Zer-koh-nee-am
Appearance: Silvery white
Mass Number: 91
Standard Atomic weight: 91.225 g/mol
Atomic number (Z): 40
Electrons: 40
Protons: 40
Neutrons: 51
Period: 5
Group: 4
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N10, O2
Electron configuration: 1s22s22p63s23p63d104s24p64d25s2

Thermal Properties of Zirconium
Phase: Solid
Melting point: 2128 K (1855 oC, 3371 oF)
Boiling point: 4650 K (4377 oC, 7911 oF)
Debye temperature: 291 K (17.85 oC, 64.13 oF)
Fusion heat: 14 kJ/mol
Vaporization heat: 591 kJ/mol
Specific heat: 278 J/(kg K)
Molar heat capacity: 25.36 J/(mol.K)
Thermal expansion: 5.7 μm/(m∙K)
Thermal conductivity: 22.6 W/(m∙K)
Electrical properties of Zirconium
Electrical conductivity: 2.4×106 S/m
A Electrical resistivity: 421 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.61 K (-272.54 oC, -458.57 oF)
Magnetic Properties of Zirconium
Magnetic type: Paramagnetic
Volume magnetic susceptibility: 0.000109
Mass magnetic susceptibility: 16.8×10-9 m3/kg
Molar magnetic susceptibility: 1.53×10-9 m3/mol
Physical Properties of Zirconium
Density: 6.52 g/cm3 (In solid) 5.8 g/cm3 (In Liquid)
Molar volume: 0.000014011 m3/mol
Young’s modulus: 88 GPa
Shear modulus: 33 GPa
Mohs Hardness: 5.0
Bulk modulus: 91.1 GPa
Poisson ratio: 0.34
Vicker hardness: 820-1800 MPa
Brinell hardness: 640-1880 MPa
Sound Speed: 3800 m/s
Atomic Properties of Zirconium
Oxidation states: 4, 3, 2,1, -2
Valence Electrons: 4d2 5s2
Ion charge: Zr4+
The ionization potential of an atom: 6
Ionization energies: 1st: 640.1 kJ.mol 2nd: 1270 kJ/mol 3rd: 2218 kJ/mol
Ionic radius: 72 pm
Atomic radius: empirical: 160 pm
Van der Waals: 186
Covalent radius: 175±7 pm
Filling Orbital: 4d2
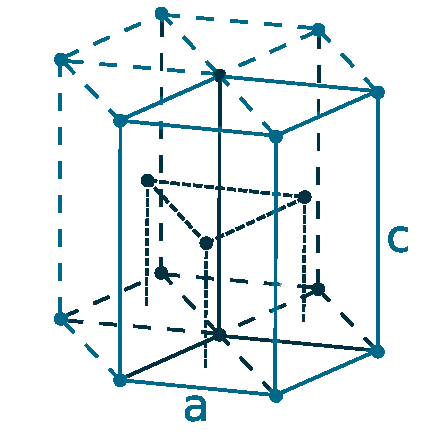
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 323.2, 323.2, 514.7 pm
Grid parameters: a=3.231 Å c=5.148 Å
Attitude c/a: 1.593
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Zirconium
Electronegativity: pauling scale: 1.33
Valence: +4
Electron affinity: 41.1 kJ/mol
Nuclear Properties of Zirconium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3F2
Neutron cross section (Brans): 0.184
Neutron Mass Absorption: 0.00066
Isotopes: 88zr 89Zr 90Zr 91Zr 92Zr 93Zr 94Zr 96Zr
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 88Zr | Syn | – | 83.4 d |
| 89Zr | Syn | – | 78.4 h |
| 90Zr | 51.45 | 89.905 | Stable |
| 91Zr | 11.22 | 90.906 | Stable |
| 92Zr | 17.15 | 91.905 | Stable |
| 93Zr | Trace | – | 1.53×106 y |
| 94Zr | 17.38 | 93.906 | Stable |
| 96Zr | 2.80 | 95.908 | 2.0×1019 |
Chemical Reactions of Zirconium
Zirconium doesn’t react with air under normal condition, But If burn with oxygen than It form:
Zr (s) + O2 (g) → ZrO2
It reacts with water at high temperatures, producing hydrogen gas:
Zr + 2 H2O → ZrO2 + 2 H2
React with Halogens when heated to form zirconium (IV) halides:
Zr (s) + 2 F2 (g) → ZrF4 (s) [white] (Zirconium (IV) fluoride)
Zr (s) + 2 Cl2 (g) → ZrCl4 (s) [white] (zirconium (IV) chloride)
Zr (s) + 2 Br2 (g) → ZrBr4 (s) [white] (zirconium (IV) bromide)
Zr (s) + 2 I2 (g) → ZrI4 (s) [white] (zirconium (IV) iodide)
Zirconium History
Naming: After zircon, zargun (meaning “Gold-colored)
Discovery: Martin Heinrich Klaproth (1789)
First isolation: Jons Jakob Berzelius (1824)
Zirconium Uses
Zirconium is used in removal of residual gases from vacuum tubes, and as an alloying agent in steel, in surgical instruments, photographic flash bulbs, rayon spinnerets, explosive primers, lamp filaments, etc.
Zirconium used in alloys such as Zircaloy (98% Zr & 2% Sn, Fe, Cr, Nb, Ni), which is used in Nuclear energy application because of it does not absorb neutrons.
It is an ideal material for use in nuclear power station, where it is used more than 90% in this way.
Some Nuclear reactors have more than 100,000 metres of zirconium alloy tubing.
With Niobium, zirconium is superconductive at low temperatures and is used to make superconductive magnets, which offer direct large-scale generation of electric power.
Zirconium is used extensively by the chemical industry where metal is protected by a thin oxide layer, making it exceptionally resistant to corrosion by acids, alkalis and seawater.
Zircon (ZrSiO4) is a natural semi-precious gemstone and has a high index of refraction that found in a variety of colours.
Zircon mixed with praseodymium (Pr) or vanadium (V) makes blue and yellow pigments for glazing pottery.
Cubic zirconia (crystalline form of zirconium dioxide) is a synthetic gemstone, The colourless stones, when cut, resemble diamonds.
The impure oxide, zirconia (ZrO2) is used in ultra-strong ceramics as a refractory material, which make Laboratory crucibles that will withstand heat shock, furnace linings, foundry bricks, abrasives and by the glass and ceramics industries.
It is so strong and flexible, that is also used in antiperspirants, cosmetics, food packaging and to make microwave filters.
Zirconia is a component in some abrasives, such as grinding wheels and sandpaper.
Biological role: It has Low-toxicity
Abundance of Zirconium
Zirconium is produced from the mineral zircon (ZrSiO4) and baddeleyite, and other 30 mineral species.
It has been identified in the sun and meteorites by Analysis of lunar rock samples, which obtained during the various Apollo missions to the moon, show a surprisingly high zirconium oxide content, compared with terrestrial rocks.
Zirconium metal is produced commercially by firstly converting zircon to zirconium chloride, and then reducing the chloride with magnesium.
Annual world wide production is around 9,00,000 tons.
5×10-6% (In Universe)
66×10-5% (In Meteorites)
4×10-6% (In Sun)
0.013% (In Earth’s Crust)
2.6×10-9% (In Oceans)
5×10-6% (In Humans)


World’s Top 3 producers of Zirconium
1) Australia
2) South Africa
3) China
World’s Top 3 Reserve holders of Zirconium
1) Australia
2) South Africa
3) Ukraine
#Zirconium


