41 Nb (Niobium)

Niobium is a soft, malleable, ductile, shiny, gray-white metal, and it takes on a bluish cast when oxidize in air at 200°C.
When Niobium processed at even moderate temperatures It must be placed in a protective atmosphere, because it tends to react with oxygen, carbon, halogens (F, Cl, Br, I) , nitrogen, and sulfur.
The metal is inert (idle) to acids, even to Aqua regia (mixture of nitric acid and hydrochloric acid) at room temperatures, but is attacked by hot, concentrated acids, and especially by alkalis and oxidizing agents.

Identity
CAS Number: CAS7440-03-1
CID Number: CID23936
RTECSQT9900000
CONTENT INDEX
Basic Properties of Niobium
Pronunciation: Ny-oh-bee-am
Appearance: Gray Metallic, bluish when oxidized
Mass Number: 93
Standard Atomic weight: 92.906 g/mol
Atomic number (Z): 41
Electrons: 41
Protons: 41
Neutrons: 52
Period: 5
Group: 5
Block: d
Element category: Transition metal
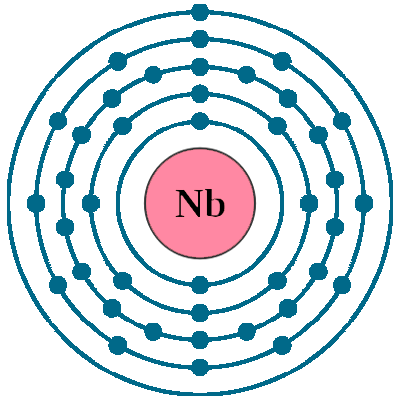
Electrons per shell: K2, L8, M18, N12, O1
Electron configuration: 1s22s22p63s23p63d104s24p64d45s1
Thermal Properties of Niobium
Phase: Solid
Melting point: 2750 K (2477 oC, 4491 oF)
Boiling point: 5017 K (4744 oC, 8571 oF)
Debye temperature: 275 K (1.85 oC, 35.33 oF)
Fusion heat: 30 kJ/mol
Vaporization heat: 689.9 kJ/mol
Specific heat: 265 J/(kg K)
Molar heat capacity: 24.60 J/(mol.K)
Thermal expansion: 7.3 μm/(m∙K)
Thermal conductivity: 53.7 W/(m∙K)
Electrical properties of Niobium
Electrical conductivity: 6.7 x106 S/m
A Electrical resistivity: 152 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 9.25 K (-263.9 oC, -443.02 oF)
Magnetic Properties of Niobium
A Magnetic type: Paramagnetic
Volume magnetic susceptibility: 0.000237
Mass magnetic susceptibility: 27.6×10-9 m3/kg
Molar magnetic susceptibility: 2.56×10-9 m3/mol
Physical Properties of Niobium
Density: 8.57 g/cm3 (In solid)
Molar volume: 0.000010841 m3/mol
Young’s modulus: 105 GPa
Shear modulus: 38 GPa
Mohs Hardness: 6.0
Bulk modulus: 170 GPa
Poisson ratio: 0.40
Vicker hardness: 870-1320 MPa
Brinell hardness: 735-2450 MPa
Sound Speed: 3480 m/s
Atomic Properties of Niobium
Oxidation states: 5, 4, 3, 2, 1, -1, -3
Valence Electrons: 4d4 5s1
Ion charge: Nb5+ Nb3+
Ionization energies: 1st: 544.5 kJ.mol 2nd: 1070 kJ/mol 3rd: 2260 kJ/mol
Ionic radius: 69 pm
Atomic radius: empirical: 146 pm
Van der Waals: 207 pm
Covalent radius: 164±6 pm
Filling Orbital: 4d4
Crystal structure: Body-centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 330.1, 330.1, 330.1 pm
Grid parameters: a=3.301 Å
Space Group Name: Im_3m
Space Group Number: 229

Reactivity of Niobium
Electronegativity: pauling scale: 1.6
Valence: +5
Electron affinity: 86.1 kJ/mol
Nuclear Properties of Niobium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 6D1/2
Neutron cross section (Brans): 1.15
Neutron Mass Absorption: 0.0004
Isotopes: 90Nb 91Nb 91mNb 92m1Nb 92Nb 93Nb 93mNb 94Nb 95Nb 95mNb 96Nb
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 90Nb | Syn | – | 15 h |
| 91Nb | Syn | – | 680 y |
| 91mNb | Syn | – | 61 d |
| 92m1Nb | Syn | – | 10 d |
| 92Nb | Trace | – | 3.47×107 y |
| 93Nb | 100 | 92.906 | Stable |
| 93mNb | Syn | – | 16 y |
| 94Nb | Trace | – | 20.3×103 y |
| 95Nb | Syn | – | 35 d |
| 95mNb | Syn | – | 4 d |
| 96Nb | Syn | – | 24 h |
Chemical Reactions of Niobium
The metal reacts with all Halogens:
2 Nb (s) + 5 F2 (g) → NbF5 (s) [white] (Niobium (V) fluoride)
2 Nb (s) + 5 Cl2 (g) → NbCl5 (l) [yellow] (Niobium (V) chloride)
2 Nb (s) + 5 Br2 (g) → NbBr3 (s) [Orange] (Niobium (V) bromide)
2 Nb (s) + 5 I2 (g) → NbI5 (s) [Brass coloured] (Niobium (V) iodide)
Production:
After the separation from the other minerals, Tantalum (Ta2O5)and Niobium (Nb2O5)are obtained that React with hydrofluoric acid and Form:
Ta2O5 + 14 HF → 2 H2[TaF7] + 5 H2O
Nb2O5 + 10 HF → 2 H2[NbOF5] + 3 H2O
Niobium and Tantalum fluorides:
Precipitated by the addition of potassium fluoride to produce a potassium fluoride complex:
H2[NbOF5] + 2 KF → K2[NbOF5]↓ + 2 HF
Or
precipitated with ammonia as the pentoxide:
2 H2[NbOF5] + 10 NH4OH → Nb2O5↓ + 10 NH4F + 7 H2O
In the Aluminothermic reaction, a mixture of Iron oxide and Niobium oxide is reacted with aluminium, and given Aluminium oxide and Ferroniobium:
3 Nb2O5 + Fe2O3 + 12 Al → 6 Nb + 2 Fe + 6 Al2O3
Ferroniobium contains 60 to 70 % niobium.
Niobium History
Naming: After Niobe in Greek mythology, daughter of Tantalus (tantalum)
Discovery: Charles Hatchett (1801)
First isolation: Christian Wilhelm Blomstrand (1864)
Recognized as a distinct element by: Heinrich Rose (1844)
Niobium Uses
Niobium is used in alloys of stainless steel to improves the strength of the alloys, particularly at low temperatures, used in jet engines and rockets, in cutting tools, in arc-welding rods, beams and girders for buildings and oil rigs, and oil and gas pipelines.
Niobium-tin and Niobium –titanium alloys has superconducting properties and It is used in superconducting magnets for particle accelerators, MRI scanners and NMR (nuclear magnetic resonance) equipment.
Niobium alloys are used in surgical implants because they do not react with human tissue.
Niobium is also commonly used for jewelry.
Niobium oxide compounds are added to glass to increase the refractive index, which allows corrective glasses to be made with thinner lenses.
Abundance of Niobium
Main source of this element is the mineral Columbite ((Fe,Mn)(Nb,Ta)2O6), It also contains tantalum and the two elements are mined together. Other ores are niobite-tantalite, parochlore, and euxenite.
Large deposits of niobium have been found associated with carbonatites (carbon-silicate rocks).
Certain amount of niobium is also produced as a by-product of tin extraction.
Annual world wide production is around 70,000 tons.
2×10-7% (In Universe)
1.9×10-5% (In Meteorites)
4×10-7% (In Sun)
0.0017% (In Earth’s Crust)
1×10-10% (In Oceans)


World’s Top producers of Niobium
1) Brazil
2) Canada
World’s Top Reserve holders of Niobium
1) Brazil
2) Canada
#Niobium