37 Rb (Rubidium)

Rubidium is a soft, silvery-white metallic, and one of the most electropositive element
It can be liquid at ambient temperature, but only on a hot day given that its melting point is about 40°C.
It ignites spontaneously in air and reacts violently in water, setting fire to the liberated hydrogen.
Its flame is yellowish-violet colour.
As with other alkali metals, it forms amalgams with mercury and it alloys with gold, sodium, cesium, and potassium.
It must be kept under a dry mineral oil or in a vacuum or inert atmosphere.

Identity
CAS Number: CAS7440-17-7
CID Number: CID5357696
DOT Hazard Class: 4.3
DOT Number: 1423
RTECS Number: RTECSVL8500000
CONTENT INDEX
Basic Properties of Rubidium
Pronunciation: Roo-bid-ee-am
Appearance: Grey white
Mass Number: 85
Standard Atomic weight: 85.467 g/mol
Atomic number (Z): 37
Electrons: 37
Protons: 37
Neutrons: 48
Period: 5
Group: 1
Block: s
Element category: Alkali metal
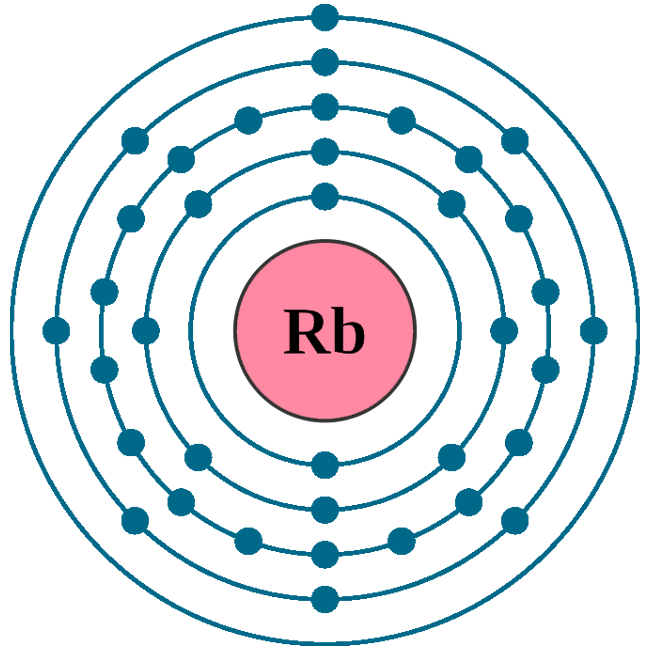
Electrons per shell: K2, L8, M18, N8, O1
Electron configuration: 1s22s22p63s23p63d104s24p65s1
Thermal Properties of Rubidium
Phase: Solid
Melting point: 312.45 K (39.30 oC, 102.74 oF)
Boiling point: 961 K (688 oC, 1270 oF)
Debye temperature: 56 K (-217.15 oC, -358.87 oF)
Triple point: 312.41 K (39.26 oC, 102.668 oF)
Critical Temperature: 2093 K (1819.85 oC, 3307.73 oF)
Critical pressure: 16 Mpa (157.9 Atm)
Fusion heat: 2.19 kJ/mol
Vaporization heat: 69 kJ/mol
Specific heat: 364 J/(kg K)
Molar heat capacity: 31.060 J/(mol.K)
Thermal expansion: 90 μm/(m∙K)
Thermal conductivity: 58.2 W/(m∙K)
Electrical properties of Rubidium
Electrical conductivity: 8.3×106 S/m
A Electrical resistivity: 128 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Rubidium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +17×10-6 cm3/mol
Volume magnetic susceptibility: 0.00000398
Mass magnetic susceptibility: 2.6×10-9 m3/kg
Molar magnetic susceptibility: 0.222×10-9 m3/mol
Physical Properties of Rubidium
Density: 1.532 g/cm3 (In solid) 1.46 g/cm3 (In Liquid at M.P)
Molar volume: 0.000055788 m3/mol
Young’s modulus: 2.4 GPa
Mohs Hardness: 0.3
Bulk modulus: 2.5 GPa
Brinell hardness: 0.216 MPa
Sound Speed: 1300 m/s
Atomic Properties of Rubidium
Oxidation states: +1, -1
Valence Electrons: 5s1
Ion charge: Rb+ Sm2+
The ionization potential of an atom: 4.16
Ionization energies: 1st: 403 kJ.mol 2nd: 2632.1 kJ/mol 3rd: 3859.4 kJ/mol
Ionic radius: 152 pm
Atomic radius: empirical: 248 pm
Van der Waals: 303 Pm
Covalent radius: 220±9 pm
Filling Orbital: 5s1
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 558.5, 558.5, 558.5 pm
Grid parameters: a=5.585 Å
Space Group Name: lm_3m
Space Group Number: 229

Reactivity of Rubidium
Electronegativity: pauling scale: 0.82
Valence: +1
Electron affinity: 46.9 kJ/mol
Nuclear Properties of Rubidium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 0.38
Neutron Mass Absorption: 0.0003
Isotopes: 83Rb 84Rb 85Rb 86Rb 87Rb
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 83Rb | Syn | – | 86.2 d |
| 84Rb | Syn | – | 32.9 d |
| 85Rb | 72.17 | 84.912 | Stable |
| 86Rb | Syn | – | 18.7 d |
| 87Rb | 27.83 | 86.909 | 4.9×1010 y |
Chemical Reactions of Rubidium
Surface of The Rubidium tarnishes, when reacts with oxygen and moisture from the air under normal condition. If heated then rubidium is oxidized to rubidium superoxide, RbO2.
Rb (s) + O2 (g) → RbO2 (s) [dark brown]
Rubidium reacts very rapidly with water, and forming colourless solution of rubidium hydroxide and hydrogen gas.
2 Rb (s) + H2O (l) → 2 RbOH (aq) + H2 (g) [colourless]
Reacts vigorously with the Halogens, and forming Rubidium halides:
2 Rb (s) + F2 (g) → RbF (s) (Rubidium (l) fluoride)
2 Rb (s) + Cl2 (g) → RbCl (s) (Rubidium (l) chloride)
2 Rb (s) + Br2 (g) → RbBr (s) (Rubidium (l) bromide)
2 Rb (s) + I2 (g) → RbI (s) (Rubidium (l) iodide)
Rubidium dissolves readily in dilute sulphuric acid (H2SO4), and forming solutions containing the Rb(I) ion together with hydrogen gas (H2).
2 Rb (s) + H2SO4 → 2 Rb+ (aq) + SO42- (aq) + H2 (g)
Rubidium doesn’t react with acids under normal condition.
Rubidium History
Naming: Latin: rubidus (red); the color its salts impart to flames.
Discovery: Robert Bunsen and Gustav Kirchhoff (1861)
First isolation: George de Hevesy
Rubidium Uses
Rubidium and its salts or components have few commercial uses.
Because of rubidium can be easily ionized, it has been considered for use in “ion engines” for space vehicles, but was found to be less effective than caesium.
It has also been proposed for use as a working fluid for vapour turbines and in thermoelectric generators.
Rubidium is used as a getter (removal of residual gases) in vacuum tubes, as a component of photocells.
Rubidium salts or components are used in making of special glasses, and in fireworks to give them a purple colour (by Rubidium nitrate).
Rubidium silver iodide (RbAg4I5) has the highest room conductivity of any known ionic crystal and At 20°C its conductivity is about the same as dilute sulfuric acid, So it is use in thin film batteries and other applications.
Biological role
It is Non-Toxic.
Because of its chemical similarity to potassium we absorb it from our food, and the average person has stores about half a gram (a 70 kg person contains on average 0.36 g of rubidium).
It is slightly radioactive and so has been used to locate brain tumours, as it collects in tumours but not in normal tissue.
Abundance of Rubidium
ARubidium occurs in the minerals pollucite, carnallite, leucite, and zinnwaldite, which contains traces up to 1% in the form of the oxide.
It is recovered commercially from lepidolite (contains about 0.3% to 3.5% Rb).
Potassium minerals (found at Searles Lake, California) and potassium chloride recovered from the brines (In Michigan) also contain rubidium and are another commercial source.
It is also found along with cesium in the extensive deposits of pollucite at Bernic Lake, Manitoba.
Rubidium compounds world wide production is around 2 to 4 tons per year.
10×10-7% (In Universe)
32×10-5% (In Meteorites)
30×10-7% (In Sun)
0.006% (In Earth’s Crust)
0.000012% (In Oceans)
0.00046% (In Humans)

#Rubidium






