03 Li (Lithium Element)

CONTENT INDEX
About Lithium Element
Pure Lithium element is a silver white, soft material. It has the lowest density of all metals, which makes it the Lighest soild element.
Like the other alkali metals, Pure Lithium element is highly flammable and slightly explosive when exposed to air and especially water.
It reacts vigorously with water, but not as vigorously as sodium.
While reacts with water It forms highly flammable hydrogen gas and corrosive fumes of Lithium hydroxide (LiOH).
When the Lithium placed over a flame, this metal gives off a beautiful crimson color but when it burns strongly, the flame becomes a dazziling white.
The element surface becomes coated with a mixture of Lithium hydroxide (LiOH), Lithium nitride (Li3N), and Lithium carbonate (Li2CO3).
The element Reacts violently with strong oxidants, acids and many compounds (halons, halogens, hydrocarbons, concrete, sand and asbestos) causing fire and explosion hazard.
Metallic Lithium is soluble in short chain aliphatic amines, like Ethylamine (C2H5NH2), but insoluble in hydrocarbons.
So it should be stored in a Non-reactive liquid hydrocarbon such as Naphtha (flammable liquid hydrocarbon mixture).

Identity
CAS Number: CAS7439-93-2
CID Number: CID3028194
DOT Hazard Class: 4.3
DOT Number: 1415
RTECS Number: RTECSOJ5540000
Properties of Lithium Element
Basic Properties of Lithium
Pronunciation: Lith-ee-am
Appearance: Silvery-white
Mass Number: 7
Standard Atomic weight: 6.94 g/mol
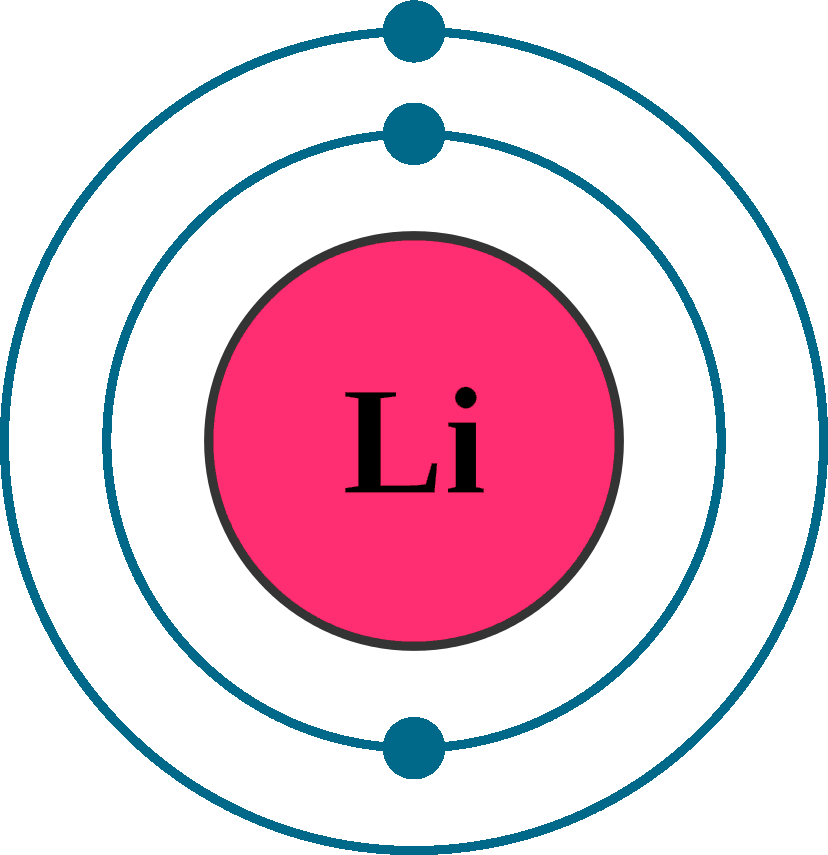
Atomic number (Z): 3
Electrons: 3
Protons: 3
Neutrons: 4
Period: 2
Group: 1
Block: S
Element category: Alkali metal
Electrons per shell: K2, L1
Electron configuration: 1s22s1
Thermal Properties of Lithium
Phase: Solid
Melting point: 453.65 K (180.50 oC, 356.90 oF)
Boiling point: 1603 K (1330 oC, 2426 oF)
Debye temperature: 400 K (126.85 oC, 260.33 oF)
Critical temperature: 3220 K (2946.85 oC, 5336 oF)
Critical pressure: 67 MPa (661 Atm)
Fusion heat: 3 kJ/mol
Vaporization heat: 136 kJ/mol
Specific heat: 3570 J/(kg K)
Molar heat capacity: 24.861 J/(mol.K)
Thermal expansion: 46 μm/(m∙K)
Thermal conductivity: 84.8 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Lithium
Electrical conductivity: 11×106 S/m
Electrical resistivity: 92.8 nΩ∙m
Electrical type: Conductor
Critical point (Superconducting point): N/A
Magnetic Properties of Lithium
Magnetic type: Paramagnetic
Curie point: N/A
Magnetic susceptibility (xmol): +14.2×10-6 cm3/mol
Volume magnetic susceptibility: 0.000000337
Mass magnetic susceptibility: 6.3×10-9 m3/kg
Molar magnetic susceptibility: 0.178×10-9 m3/mol
Physical Properties of Lithium
Density: 0.534 g/cm3 (In solid) 0.512 g/cm3 (In Liquid at M.P)
Molar volume: 0.00001297 m3/mol
Young’s modulus: 4.9 GPa
Shear modulus: 4.2 GPa
Mohs Hardness: 0.6
Bulk modulus: 11 GPa
Poisson ratio: N/A
Vicker hardness: N/A
Brinell hardness: 5 MPa
Sound Speed: 6000 m/s
Atomic Properties of Lithium
Oxidation states: +1
Valence Electrons: 2s1
Ion charge: Li+
The ionization potential of an atom: 5.37 eV
Ionization energies: 1st: 520.2 kJ.mol 2nd: 7298.1 kJ/mol 3rd: 11815 kJ/mol
Ionic radius: 76 pm
Atomic radius: 152 pm (empirical)
Van der Waals: 182 Pm
Covalent radius: 128±7 pm
Filling Orbital: 2s1
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 349, 349, 349 pm
Grid parameters: a= 3.490 Å
Attitude c/a: 7.25
Space Group Name: Im_3m
Space Group Number: 229

Reactivity of Lithium
Electronegativity: 0.98 (pauling scale)
Valence: +1
Electron affinity: 59.5 kJ/mol
Nuclear Properties of Lithium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 71
Neutron Mass Absorption: N/A
Isotopes: 6Li 7Li
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 6Li | 5 | 6.020 | Stable |
| 6Li | 95 | 7.015 | Stable |
Chemical Reactions of Lithium Element
Reaction with Air
The metal reacts slowly with Oxygen (O2) at normal temperature, and forming Lithium oxide (Li2O). If burned the metal, a minor amount of Peroxide (Li2O2) is produces as well.
4 Li (s) + O2 (g) → 2 Li2O (s) [white]
2 Li (s) + O2 (g) → Li2O2 (s) [white]
Reaction with Water
Lithium reacts slowly with water, and forming a colorless solution of Lithium hydroxide (LiOH) and hydrogen gas (H2).
2 Li (s) + 2 H2O (l) → 2 LiOH (aq) + H2 (g)
Reaction with Halogens
The metal reacts with the halogens, and forming the corresponding lithium halides.
2 Li (s) + F2 (g) → 2 LiF (s) (Lithium fluoride)
2 Li (s) + Cl2 (g) → 2 LiCl (s) (Lithium Chloride)
2 Li (s) + Cl2 (g) → 2 LiBr (s) (Lithium Bromide)
2 Li (s) + I2 (g) → 2 LiI (s) (Lithium Iodide)
Reaction with Acids
Lithium Dissolves readily in dilute sulphuric acid (H2SO4), and forming Lithium (Li) ions and hydrogen gas (H2).
2 Li (s) + H2SO4 (aq) → 2 Li+ (aq) + SO42- (aq) + H2 (g)
Reaction with Hydrogen
Lithium reacts with hydrogen, and forming Lithium hydride (LiH).
2 Li (s) + H2 (g) → 2 LiH (s)
Reaction with Neutrons
The production of Tritium (Radioactive form of hydrogen) from Lithium Deuteride (LiD).
Li36 + n → He24 + H13
This reaction produces the tritium on the spot.
Lithium History
Naming: Greek: lithos (stone)
Discovery: Johan August Arfwedson (1817), at Stockholm (Sweden)
First Isolation: William Thomas Brande (1821)
Lithium Uses
Because of its highest specific heat, Lithium is used in heat transfer applications. The production of lithium metal and its compounds has increased greatly.
The metal is Largely uses in Rechargeable batteries for Laptops, Mobile phones, Digital camera, Electric vehicles and many more electronic gadgets and also in non-Rechargeable batteries for many things like Heart Pacemaker, Toys, Remotes. Clocks etc…
Lithium metal is made into alloys with aluminium, magnesium, cadmium, and copper to improving their strength and making them lighter.
A Magnesium-Lithium alloy is used for Armour Plating, and Aluminium-lithium alloys are used in bicycle frames, high-speed trains, and to make high performance Aircraft parts.
Lithium is sometimes used as Lithium oxide (Li2O) in special glasses and ceramics, like the glass used in the 200-inch telescope at Mt. Palomar contains lithium as a minor ingredient.
Lithium hydroxide (LiOH) is a white powder, which is employed to extract carbon dioxide from the air in spacecraft and submarines. It is commercially available in anhydrous form and as the monohydrate (LiOH.H2O).
Lithium deuteride (LiD) is used in Nuclear weapons to fusion explosion.
Lithium hydride (LiH) is used as a means of storing hydrogen for use as a fuel.
Lithium chloride (LiCl) and Lithium bromide (LiBr) are extremely hygroscopic (readily attracts water) materials known, and are used in Air conditioning and industrial drying systems.
Lithium stearate (LiO2C(CH2)16CH3 or C18H35LiO2) is commonly used as an all-purpose high-temperature lubricant.
Lithium salts such as Lithium carbonate (Li2CO3), Lithium citrate (Li3C6H5O7), and Lithium orotate (LiC5H3N2O4) are mood stabilizers, that are used in the treatment of Bipolar disorder (Manic depression) and other mental illness conditions.
The main industrial use of lithium is in lithium stratum form (igneous rock), as lubricant grease’s thickener.
Biological Role of Lithium
Lithium is Toxic, except in very small doses.
The metal is also corrosive and it requires special handling to avoid skin contact.
Lithium is Flammable, and Many reactions may cause fire or explosion. It gives off irritating or toxic fumes in a fire. It has a risk of fire and explosion on contact with combustible substances (catch fire easily) and water.
Effects of Exposure to Lithium by
Inhalation: effect will occur as Sore throat, burning sensation, cough, Shortness of breath, Laboured breathing, Lung oedema, However Symptoms may be delayed.
Skin: effects are Redness, Blisters, Pain, Skin burns.
Eyes: Redness, Pain, Severe deep burns.
Ingestion: Abdominal pain, Abdominal cramps, Nausea, Burning sensation, Shock or collapse, Vomiting, Weakness.
Heating the substance may cause violent combustion or explosion, even may spontaneously ignite on contact with air when finely dispersed (spread over a wide area). Toxic fumes are formed while heating.
Lithium Sources
Because of its high reactivity, Lithium does not occur freely in Nature, it is found combined in small amount in almost all igneous rocks and in many mineral springs.
Lepidolite (K(Li,Al,Rb)2(Al,Si)4O10(F,OH)2), Petalite (LiAlSi4O10), Spodumene (Kunzite, LiAlSi2O6), and Amblygonite (Li,Na)Al(PO4)(F,OH) are the most important minerals containing lithium.
Lithium is recoverd from brine pools in Nevada (USA), and Most lithium is presently produced in chile, from brines that yield lithium carbonate when treated with sodium carbonate. Large deposits of quadramene are found in North Carolina.
The metal is produced by the electrolysis of molten lithium chloride (LiCl) and potassium chloride (KCl).
Lithium is present in The Earth’s crust in 65 ppm (parts per million).




Annual world wide production is around 50,000-80,000 tonnes, and World wide Reserve is around 16,000,000 tonnes.
6×10-7% (In Universe)
0.00017% (In Meteorites)
6×10-9% (In Sun)
0.0017% (In Earth’s Crust)
18×10-6% (In Oceans)
3×10-6% (In Humans)
World’s Top 3 producers of Lithium
1) Australia
2) Chile
3) China
World’s Top 3 Reserve holders of Lithium
1) Chile
2) China
3) Australia
Lithium Price
Pure (99.995%) metal price is around $270-$300 per KG (KiloGram)
Lithium Element Database
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
→ Electrons (ESTAR)
→ Helium Ions (ASTAR)
→ Protons (PSTAR)
Nuclear Physics Data
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements



Mining Issues and minerals knowledge