58 Ce (Cerium)

Cerium is a Soft, malleable, ductile, iron-grey lustrous metal.
It is very reactive, and it oxidizes very readily in the air, especially in moist air.
It decomposes slowly in cold water and rapidly in hot water.
Alkali solutions, dilute and concentrated acids attack on the metal rapidly.
The pure metal can burn when heated or scratched with a knife.

Identity
CAS Number: CAS7440-45-1
CID Number: CID23974
DOT Hazard Class 4.1
DOT Numbers: 1333
CONTENT INDEX
Basic Properties of Cerium
Pronunciation: Seer-ee-am
Appearance: Silvery white
Mass Number: 146
Standard Atomic weight: 140.116 g/mol
Atomic number (Z): 58
Electrons: 58
Protons: 58
Neutrons: 82
Period: 6
Block: f
Element category: Lanthanide
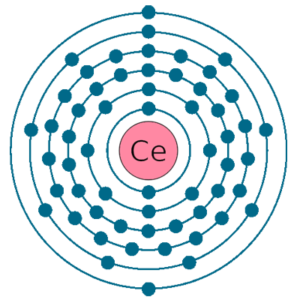
Electrons per shell: K2, L8, M18, N19, O9, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f15d16s2
Thermal Properties of Cerium
Phase: Solid
Melting point: 1068 K (795 oC, 1463 oF)
Boiling point: 3716 K (3443 oC, 6229 oF)
Fusion heat: 5.46 kJ/mol
Vaporization heat: 398 kJ/mol
Specific heat: 192 J/(kg K)
Molar heat capacity: 26.94 J/(mol.K)
Thermal expansion: γ, poly: 6.3 μm/(m∙K)
Thermal conductivity: 11.3 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 12.5 K (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Cerium
Electrical conductivity: 1.1×106 S/m
A Electrical resistivity: β, poly: 828 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.022 K (-273.13 oC, -459.63 F)
Magnetic Properties of Cerium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): (β) +2450.0×10-6 cm3/mol
Volume magnetic susceptibility: 0.0014716
Mass magnetic susceptibility: 220×10-9 m3/kg
Molar magnetic susceptibility: 30.82×10-9 m3/mol
Physical Properties of Cerium
Density: 6.770 g/cm3 (In solid) 6.55 g/cm3 (In Liquid)
Molar volume: 0.00002095 m3/mol
Young’s modulus: γ form: 33.6 GPa
Shear modulus: γ form: 13.5 GPa
Mohs Hardness: 2.5
Bulk modulus: γ form: 21.5 GPa
Poisson ratio: γ form: 0.24
Vicker hardness: 210-470 MPa
Brinell hardness: 186-412 MPa
Sound Speed: 2100 m/s
Atomic Properties of Cerium
Oxidation states: 4,3,2,1
Valence Electrons: 4f2 6s2
Ion charge: Ce3+
Ionization energies: 1st: 534.4 kJ.mol 2nd: 1050 kJ/mol 3rd: 1949 kJ/mol
Ionic radius: 103.4 pm
Atomic radius: 235 pm (Van der Waals)
Covalent radius: 204±9 pm
Filling Orbital: 4f2
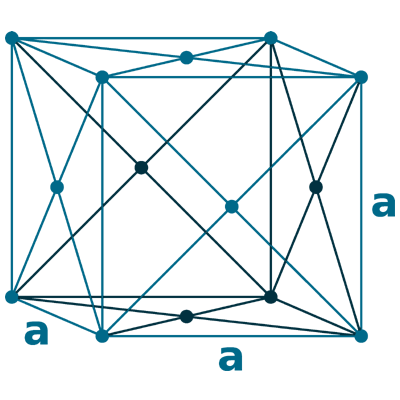
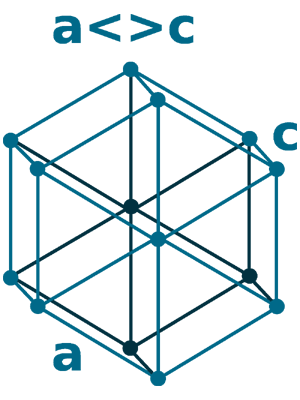
Crystal structure: δ-Ce; Body centered cubic (above 726 oC), γ-Ce; Face-centered cubic (726 oC to Room temperature), β-Ce ; Double hexagonal close-packed (Room temperature to -150 oC)
Grid parameters: 5.256 Å (Face-centered cubic)
Space Group Name: P63/mmc
Space Group Number: 194

Reactivity of Cerium
Electronegativity: pauling scale: 1.12
Valence: +4
Electron affinity: 50 kJ/mol
Nuclear Properties of Cerium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1G4
Neutron cross section (Brans): 0.6
Neutron Mass Absorption: 0.00021
Isotopes: 134Ce 136Ce 138Ce 139Ce 140Ce 141Ce 142Ce 143Ce 144Ce
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 134Ce | Syn | – | 3.16 d |
| 136Ce | 0.186 | 135.907 | Stable |
| 138Ce | 0.251 | 137.906 | Stable |
| 139Ce | Syn | – | 137.640 |
| 140Ce | 88.449 | 139.905 | Stable |
| 141Ce | Syn | – | 32.501 d |
| 142Ce | 11.114 | 141.909 | Stable |
| 143Ce | Syn | – | 33.039 d |
| 144Ce | Syn | – | 284.893 d |
Chemical Reactions
Cerium Burn readily at 150 oC to form the pale-yellow cerium (lV) oxide:
Ce + O2 → CeO2
Reacts slowly in cold water and rapidly in hot water (Produce cerium (lll) hydroxide and hydrogen gas):
2 Ce (s) + 6 H2O (l) → 2 Ce(OH)3 (aq) + 3 H2 (g)
The metal reacts with Halogens to form Trihalides:
2 Ce (s) + 3 F2 (g) → 2 CeF3 (s) [white]
2 Ce (s) + 3 Cl2 (g) → 2 CeCl3 (s) [white]
2 Ce (s) + 3 Br2 (g) → 2 CeBr3 (s) [white]
2 Ce (s) + 3 I2 (g) → 2 CeI3 (s) [yellow]
Dissolves readily in dilute sulfuric acid:
2 Ce (s) + 3 H2SO4 (aq) → 2 Ce3+ (aq) + 3 SO42−(aq) + 3 H2 (g)
Cerium History
Naming: After dwarf planet Ceres, Itself named after Roman god of agriculture
Discovery: Martin Heinrich klaproth, Jons Jakob Berzelius, Wilhelm Hisinger (1803)
First isolation: Carl gustaf Mosander (1838)
Cerium Uses
Cerium is not radioactive, but the impure cerium (contain thorium) is a radioactive.
Mischmetal Is an alloy (Containing 50% cerium, 25% lanthanum, Small amounts of Neodymium and Praseodymium), which is used in making cigarette lighter flints. Cerium is the major component of mischmetal alloy, because cerium make sparks when struck.
It is used as a core material for carbon arc lights, which is used by the motion picture industry for studio lighting and projection.
Cerium oxide (CeO2) has use as a catalyst, which is used in the walls of self-cleaning ovens to prevent the collection of cooking residue. CeO2 is also used in incandescent gas mantle for lighting.
It is used in catalytic converters to clean up exhaust of the vehicles, where It is catalyzes the reduction of nitrogen oxides (Nox) to nitrogen gas.
A Little Cerium oxide is added to the fuel, where its Nanoparticles help it burn more completely and reduce exhaust emissions.
It is useful in petroleum refining and in metallurgical and nuclear applications.
Cerium sulfide (Ce2S3) is a non-toxic compound, which is used extensively as a volumetric oxidizing agent in quantitative analysis and as a red pigment for toys, containers, household wares and crates. It replace cadmium, Due to cadmium consider environmentally undesirable.
Other uses of cerium is in Flat-screen TV, Low energy light bulbs, flood lights, and magnetic-optic compact discs.
Abundance of Cerium
It is the most abundant lanthanide metal, which is Found in various minerals like Allanite (orthite), monazite, bastnasite, cerite, and samarskite. The Most important sources are Monazite and bastnasite.
Metallic cerium is obtained by metallothermic reduction techniques(Produces high-purity cerium), such as heating cerium (lll) fluoride (Cef3) with calcium, or by the electrolysis of molten cerium chloride.
Annual world wide production is around 24000 tons.
1×10-6% (In Universe)
7.5×10-5% (In Meteorites)
4×10-7% (In Sun)
0.006% (In Earth’s Crust)
1.2×10-10% (In Oceans)
World’s Top 3 producers of Cerium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Cerium
1) China
2) CIS Countries (inc. Russia)
3) USA
#cerium


