36 Kr (Krypton Element)

Under normal conditions krypton is colourless, odourless, fairly expensive gas.
Solid krypton is a white crystalline substance.
Krypton is a “noble” gas, and it is characterised by its brilliant green and orange spectral lines.
The spectral lines of krypton are easily produced and some are very sharp.

Identity
CAS Number: CAS7439-90-9
CID Number: CID5416
DOT Hazard Class: 2.2
DOT Number: 1970
RTECS Number: RTECSOC6772500
CONTENT INDEX
Basic Properties of Krypton Element
Pronunciation: Krip-ton
Appearance: Colourless gas (exhibiting a whitish glow in an electric field)
Mass Number: 84
Standard Atomic weight: 83.798 g/mol
Atomic number (Z): 36
Electrons: 36
Protons: 36
Neutrons: 48
Period: 4
Group: 18
Block: p
Element category: Nobal gas
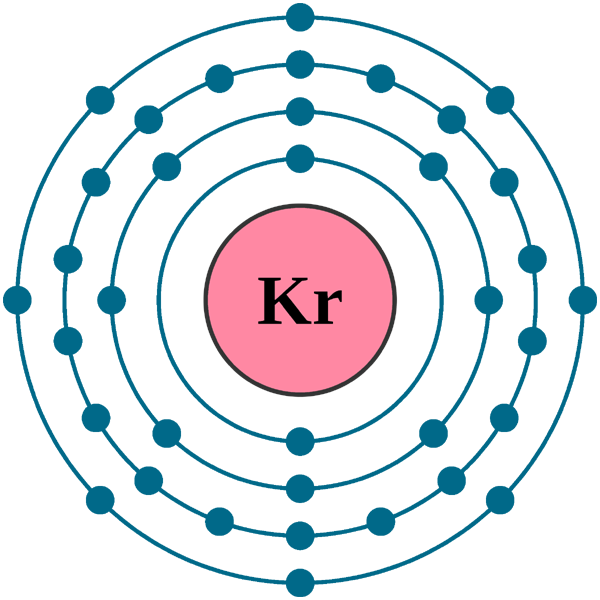
Electrons per shell: K2, L8, M18, N8
Electron configuration: 1s22s22p63s23p63d104s24p6
Thermal Properties of Krypton
Phase: Gas
Melting point: 115.78 K (-157.37 oC, -251.27 oF)
Boiling point: 119.93 K (-153.415 oC, -244.147 oF)
Debye temperature: 72 K (-201.15 oC, -330.07 oF)
Fusion heat: 1.64 kJ/mol
Vaporization heat: 9.08 kJ/mol
Specific heat: 248.05 J/(kg K)
Molar heat capacity: 20.95 J/(mol.K)
Triple point Temperature: 115.775 K (-157.375 oC, -251.275 oF)
Triple point pressure: 73.53 kPa (0.725 Atm)
Critical Temperature: 209.48 K (-63.67 oC, -82.606 oF)
Critical pressure: 5.525 MPa (54.527 Atm)
Adiabatic index: 5/3
Thermal conductivity: 9.43×10-3 W/(m∙K)
Magnetic Properties of Krypton
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -28.8×10-6 cm3/mol
Volume magnetic susceptibility: -16.5×10-9
Mass magnetic susceptibility: -4.4×10-9 m3/kg
Molar magnetic susceptibility: -0.369×10-9 m3/mol
Physical Properties of Krypton
Density: 3.749 g/cm3 (In Gas, STP) 2.413 g/cm3 (In Liquid at B.P)
Molar volume: 0.02235 m3/mol
Refractive index: 1.000427
Sound Speed: 220 m/s (In Gas), 1120 m/s (in liquid)
Atomic Properties of Krypton
Oxidation states: 2, 1, 0
Valence Electrons: 4s2 4p6
The ionization potential of an atom: 13.94
Ionization energies: 1st: 1350.8 kJ.mol 2nd: 2350.4 kJ/mol 3rd: 3565 kJ/mol
Atomic radius: 88 pm
Van der Waals: 202 Pm
Covalent radius: 116±4 pm
Filling Orbital: 4p6
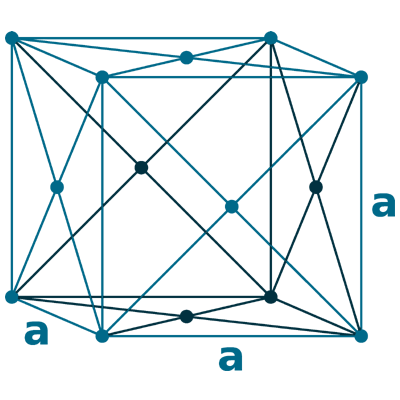
Crystal structure: Face centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 563.8, 563.8, 563.8 pm
Grid parameters: a=5.638 Å
Attitude c/a: 7.25
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Krypton
Electronegativity: pauling scale: 3.00
Valence: +2
Electron affinity: 0 kJ/mol
Nuclear Properties of Krypton Element
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 25
Neutron Mass Absorption: 0.013
Isotopes: 78Kr 79Kr 80Kr 81Kr 82Kr 83Kr 84Kr 85Kr 86Kr
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 78Kr | 0.36 | 77.920 | 9.2×1021 y |
| 79Kr | Syn | – | 35 h |
| 80Kr | 2.29 | 79.916 | Stable |
| 81Kr | Trace | – | 2.3×105 y |
| 82Kr | 11.59 | 81.913 | Stable |
| 83Kr | 11.50 | 82.914 | Stable |
| 84Kr | 56.99 | 83.911 | Stable |
| 85Kr | Syn | – | 11 y |
| 86Kr | 17.28 | 85.911 | Stable |
Chemical Reactions of Krypton Element
Krypton reacts with fluorine (F2), when it cooled to -196 °C (the boiling point of nitrogen) and zapped with an electric discharge or X-rays, Forming a Krypton (II) fluoride (KrF2):
Kr + F2 → KrF2 [krypton difluoride]
This compound decomposes when heating to room temperature.
Other halogens doesn’t react with krypton .
Krypton History
Naming: Greek: Kryptos (hidden)
Discovery: William Ramsay and Morris in London, UK
First isolation: Travers (1898)
Krypton Uses
Krypton clathrates (cage compounds) are prepared using hydroquinone (C6H6O2) and phenol (Phenolic acid, C6H5OH).
A Krypton is used commercially as a filling gas (filled with a mixture of krypton and argon) for energy-saving fluorescent lights.
It is also used in some flash lamps (photographic projection lamps) used for high-speed photography, in very high-powered electric arc lights used at airports, and in some stroboscopic-lamps, because it has an extremely fast respons to an electric current.
It is reactive enough to form some chemical compounds, Like krypton reacts with fluorine to form krypton fluoride, which is used in some lasers.
A mixture of stable and unstable isotopes of krypton is produced by slow neutron fission of uranium in nuclear reactors as Kripton-85 (most stable isotope).
It is used to detect leaks in sealed containers, to excite phosphors in light sources with no external source of energy, and in medicine to detect abnormal heart openings.
85Kr can be used for chemical analysis by imbedding the isotope in various solids, During this process, kryptonates are formed.
Kryptonate activity is sensitive to chemical reactions at the solution surface.
The metastable isotope krypton-81m is used in nuclear medicine for lung ventilation/perfusion scans.
83Kr has application in magnetic resonance imaging (MRI) for imaging airways.
The isotope 86Kr was used to define the standard measure of length, where 1 metre was defined as exactly 1,650,763.73 wavelengths of a line in the atomic spectrum of the isotope.
Radioactive krypton was used during the Cold War to estimate Soviet nuclear production by subtracting the amount of gas, that came from Western reactors from the total in the air.
Biological role Krypton Element: It is considered to be a harmless gas, it could asphyxiate if it excluded oxygen from the lungs.
Abundance of Krypton
Krypton is one of the rarest gases in the Earth’s atmosphere, it is present in the air to the extent of about 1 ppm (part per million).
The atmosphere of Mars has been found to contain 0.3 ppm of krypton.
It is extracted by distillation of air that has been cooled until it is a liquid.
World wide krypton is produced around 10 tons each year.
40×10-7% (In Universe)
1.5×10-8% (In Earth’s Crust)
2.1×10-8% (In Oceans)


