35 Br (Bromine)

Bromine is a heavy, mobile, brownish-red nonmetallic liquid element.
It is volatilizing readily or evaporates easily at room temperature to a red vapor with a strong disagreeable (suffocating) odor, resembling that of chlorine.
Bromine is readily soluble in water or carbon disulfide (CS2), forming a red solution, and it is less active chemically than chlorine and fluorine but is more active than iodine

Identity
CAS Number: CAS7726-95-6
CID Number: CID24408
DOT Hazard Class: 4.1
DOT Number: 1325
RTECS Number: RTECSEF9100000
CONTENT INDEX
Basic Properties of Bromine
Pronunciation: Broh-meen / myn
Appearance: Reddish-brown
Mass Number: 80
Standard Atomic weight: 79.904 g/mol
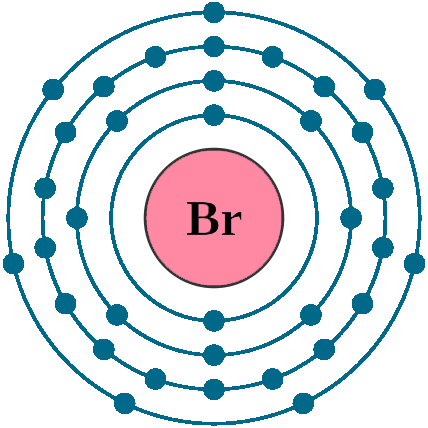
Atomic number (Z): 35
Electrons: 35
Protons: 35
Neutrons: 45
Period: 4
Group: 17
Block: p
Element category: Halogens
Electrons per shell: K2, L8, M18, N7
Electron configuration: 1s22s22p63s23p63d104s24p5
Thermal Properties of Bromine
Phase: liquid
Melting point: 265.8 K (-7.2 oC, 19 oF)
Boiling point: 332 K (58.8 oC, 137.8 oF)
Debye temperature: K ( oC, oF)
Triple point temperature: 265.90 K (-7.25 oC, 18.95 oF)
Triple point pressure: 5.8 kPa (0.057 Atm)
Critical point temperature: 588 K (314.85 oC, 598.73 oF)
Critical point pressure: 10.34 MPa (102.047 Atm)
Fusion heat: 10.571 kJ/mol
Vaporization heat: 29.96 kJ/mol
Specific heat: 947.3 J/(kg K)
Molar heat capacity: 75.69 J/(mol.K)
Thermal conductivity: 0.122 W/(m∙K)
Electrical properties of Bromine
Electrical conductivity: 1 x10-10 S/m
A Electrical resistivity: 7.8×1010 Ω∙m
A Electrical type: Insulator
Magnetic Properties of Bromine
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -56.4×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000153
Mass magnetic susceptibility: -4.9×10-9 m3/kg
Molar magnetic susceptibility: -0.783×10-9 m3/mol
Physical Properties of Bromine
Density: 3.1028 g/cm3 (In Liquid)
Molar volume: 0.00002045 m3/mol
Refractive index: 1.001132
Sound Speed: 2130 m/s
Atomic Properties of Bromine
Oxidation states: 7, 5, 4, 3, 1, -1
Valence Electrons: 4s2 4p5
Ion charge: Br–
The ionization potential of an atom: 11.8
Ionization energies: 1st: 544.5 kJ.mol 2nd: 1070 kJ/mol 3rd: 2260 kJ/mol
Ionic radius: 196 pm
Atomic radius: empirical: 120 pm
Van der Waals: 185 Pm
Covalent radius: 120±3 pm
Filling Orbital: 4p5
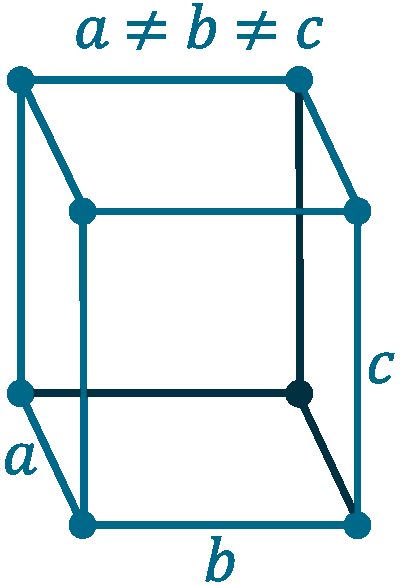
Crystal structure: Orthorhombic
Lattice angles: π/2, π/2, π/2
Lattice constant: 667, 448, 872 pm
Grid parameters: a=6.67 Å b=4.48 Å c=8.72 Å
Space Group Name: Cmca
Space Group Number: 64
Reactivity of Bromine
Electronegativity: pauling scale: 2.96
Valence: +5
Electron affinity: 324.6 kJ/mol
Nuclear Properties of Bromine
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2P3/2
Neutron cross section (Brans): 6.8
Neutron Mass Absorption: 0.002
Isotopes: 79Br 81Br
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 79Br | 51 | 78.918 | Stable |
| 81Br | 49 | 80.916 | Stable |
Chemical Reactions of Bromine
Bromine doesn’t react with Oxygen (O2) or Nitrogen (N2), But it react with Ozone (O3) at -78 oC, and Forming a Bromide (IV) oxide:
Br2 (l) + 2 O3 (g) → O2 (g) + 2 BrO2 (s) [brown]
Reacts with carbon monoxide (CO), and forming Carbonyl bromide(COBr2):
Br2 (l) + CO (g) → COBr2 (l)
Reacts with water, and forming hypobromide (BrO–):
Br2 (l) + H2O (l) ⇌ BrO– (aq) + 2 H+ (aq) + Br– (aq)
Reacts with Fluorine in gas phase, and forming BrF:
Br2 (g) + F2 (g) → 2 BrF (g)
The product is difficult to obtain pure as BrF react with itself, and forming Br2, BrF3 and BrF5:
3 BrF (g) → Br2 (l) + BrF3 (l)
5 BrF (g) → 2 Br2 (l) + BrF5 (l)
Using excess Fluorine at 150 oC, Bromine will react with fluorine, and forming BrF5:
Br2 (l) + 5 F2 (g) → 2 BrF5 (l)
Reacts with Chlorine in gas phase, and forming the unstable bromine (l) chloride:
Cl2 (g) + Br2 (g) → 2 ClBr (g)
Reacts with Iodine at room temperature, and forming bromine (I) iodide:
Br2 (l) + I2 (s) → 2 IBr (s)
Reacts with hot aqueous alkali, and forming bromated (BrO3–):
3 Br2 (g) + 6 OH– (aq) → BrO3– (aq) + 5 Br– (aq) + 3 H2O (l)
Only 1/6 th of the total bromine is converted in this reaction.
Hydrogen bromide produced by the reaction of hydrogen gas with bromine gas at 200–400 °C with a platinum catalyst:
2 P + 6 H2O + 3 Br2 → 6 HBr + 2 H3PO3
H3PO3 + H2O + Br2 → 2 HBr + H3PO4
HBr is Colourless gas at room temperature.
Bromine History
Naming: Greek: brômos (stench).
Discovery and First isolation: Antoine Jerome Balard and Carl Jacob Lowig (1825)
Discovery Location: Montpellier France / Heidelberg Germany
Bromine Uses
Bromine is used in many are as such as agricultural chemicals, insecticides, making fumigants, dyestuffs, water purification compounds, sanitizes, used as emulsifier in many citrus-flavoured soft drinks, pharmaceuticals and chemical intermediates.
A Bromine compounds can be used as flame retardants (flameproofing agents).
They are added to furniture foam, plastic casings for electronics and textiles to make them less flammable.
However, the use of bromine as a flame retardant has been phased out in the USA because of toxicity concerns.
Before leaded fuels were phased out, Bromine was used in industry to make organobromo compounds, and a major one was 1,2-di-bromoethane (C2H4Br2) an anti-knock agent for leaded gasoline.
Organobromides are used in halon fire extinguishers that are used to fight fires in places like museums, aeroplanes and tanks.
Silver bromide (AgBr) is a chemical used in film photography.
Biological role
Bromine is present in small amounts, as bromide, in all living things.
It has an irritating effect on the eyes and throat, and produces painful sores when in contact with the skin.
It presents a serious health hazard, and maximum safety precautions should be taken when handling it.
Abundance of Bromine
Bromine is significantly more abundant in the oceans, that is extracted today from seawater, which contains about 85 ppm (Parts per million).
Bromine is also obtained from natural salt lakes and brine wells in Michigan and Arkansas (USA).
It extracted economically viable at the Dead Sea (Israel), which is particularly rich in bromide ions (up to 0.5%).
The element is liberated by halogen exchange by using chlorine gas to oxidise Br– to Br2.
World wide production is around 4,00,000 tons per year.
7×10-7% (In Universe)
12×10-5% (In Meteorites)
0.0003% (In Earth’s Crust)
0.0066% (In Oceans)
0.00029% (In Humans)
World’s Top 3 producers of Bromine
1) USA
2) China
3) Israel
World’s Top 3 Reserve holders of Bromine
1) USA
2) China
3) Spain
#Bromine



This was the last page .I done here.