34 Se (Selenium)

Selenium is a non metallic chemical element exists in several allotropic forms, although three are generally recognized.
In chemical activity and physical properties it resembles sulfur and tellurium.
It can be prepared with either an amorphous or a crystalline structure.
The color of amorphous selenium is either red (in powder form) or black (in vitreous form).
Crystalline monoclinic selenium is a deep red and crystalline hexagonal selenium (the most stable variety) is a metallic gray.
Selenium burns in air and is uneffected by water, but dissolves in concentrated nitric acid and alkalis.

Identity
CAS Number: CAS7782-49-2
CID Number: CID6326970
DOT Hazard Class: 6.1
DOT Number: 2658
RTECS Number: RTECSVS7700000
CONTENT INDEX
Basic Properties of Selenium
Pronunciation: Sa-lee-nee-am
Appearance: Black, red, and gray allotropes
Allotropes: Amorphous Red Selenium, Amorphous Black Selenium, Monoclinic Selenium, Hexagonal Selenium
Mass Number: 79
Standard Atomic weight: 78.97 g/mol
Atomic number (Z): 34
Electrons: 34
Protons: 34
Neutrons: 45
Period: 4
Group: 16
Block: p
Element category: Non-metal
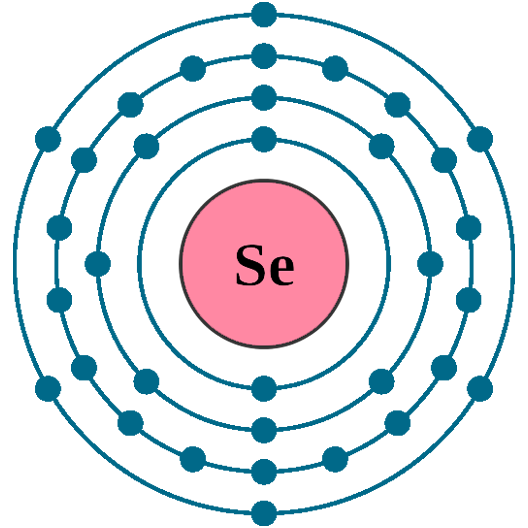
Electrons per shell: K2, L8, M18, N6
Electron configuration: 1s22s22p63s23p63d104s24p4
Thermal Properties of Selenium
Phase: Solid
Melting point: 494 K (221 oC, 430 oF)
Boiling point: 958 K (685 oC, 1265 oF)
Debye temperature: 90 K (-183 oC, -297.67 oF)
Critical Point Temperature: 1766 K (1492.85 oC, 2719.13 oF)
Critical point pressure: 27.2 MPa (268.443 Atm)
Fusion heat: gray: 6.69 kJ/mol
Vaporization heat: 95.48 kJ/mol
Specific heat: 321.2 J/(kg K)
Molar heat capacity: 25.363 J/(mol.K)
Refrective index: 1.000895
Thermal expansion: amorphous: 37 μm/(m∙K)
Thermal conductivity: amorphous: 0.519 W/(m∙K)
Magnetic Properties of Selenium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -25×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000193
Mass magnetic susceptibility: -4×10-9 m3/kg
Molar magnetic susceptibility: -0.316×10-9 m3/mol
Physical Properties of Selenium
Density: 4.81 g/cm3 (gray) 4.39 g/cm3 (alpha) 4.28 g/cm3 (vitreous)
Molar volume: 0.000016387 m3/mol
Young’s modulus: 10 GPa
Shear modulus: 3.7 GPa
Mohs Hardness: 2.0
Bulk modulus: 8.3 GPa
Poisson ratio: 0.33
Vicker hardness: MPa
Brinell hardness: 736 MPa
Sound Speed: 3350 m/s
Atomic Properties of Selenium
Oxidation states: 6, 5, 4, 3, 2, 1, -1, -2
Valence Electrons: 4s2 4p4
Ion charge: Se2-
The ionization potential of an atom: 9.5
Ionization energies: 1st: 941 kJ.mol 2nd: 2045 kJ/mol 3rd: 2973.7 kJ/mol
Ionic radius: 50 pm
Atomic radius: empirical: 120 pm
Van der Waals: 190 Pm
Covalent radius: 120±4 pm
Filling Orbital: 4p4
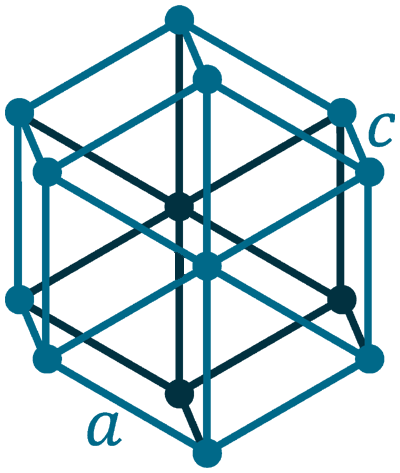
Crystal structure: Hexagonal
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 436.4, 436.4, 495.9 pm
Grid parameters: a=4.364 Å c=4.959 Å
Attitude c/a: 1.136
Space Group Name: P121/c1
Space Group Number: 14
Reactivity of Selenium
Electronegativity: pauling scale: 2.55
Valence: +6
Electron affinity: 195 kJ/mol
Nuclear Properties of Selenium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P2
Neutron cross section (Brans): 11.7
Neutron Mass Absorption: 0.0056
Isotopes: 72Se 74Se 75Se 76Se 77Se 78Se 79Se 80Se 82Se
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 72Se | Syn | – | 8.4 d |
| 74Se | 0.86 | 73.922 | Stable |
| 75Se | Syn | – | 119.8 d |
| 76Se | 9.23 | 75.919 | Stable |
| 77Se | 7.60 | 76.920 | Stable |
| 78Se | 23.69 | 77.917 | Stable |
| 79Se | Trace | – | 3.27×105 y |
| 80Se | 49.80 | 79.917 | Stable |
| 82Se | 8.82 | 81.917 | 1.08×1020 y |
Chemical Reactions of Selenium
Selenium burns in air, and forming Selenium (IV) oxide (SeO2):
Se8 (s) + 8 O2 (g) → 8 SeO2 (s)
Se (IV) oxide reacts with water, and forming selenious acid:
SeO2 (s) + H2O (l) → H2SeO3 (aq)
Reacts with Fluorine at 0 oC, and forming Se (IV) fluoride:
Se8 (s) + 15 F2 (g) → 8 SeF4 (s) [colourless] (Selenium tetra-fluoride)
If burned, forming Se (VI) fluoride:
Se8 (s) + 24 F2 (g) → 8 SeF6 (l) [orange] (Selenium hexa-fluoride)
Se (Preferably suspended in CS2) reacts with chlorine and bromine, forming:
Se8 + 4 Cl2 → 4 Se2Cl2 (l) [orange] (di-selenium di-chloride)
Se8 + 4 Br2 → 4 Se2Br2 (l) [orane] (di-selenium di-bromide)
Reacts under controlled condition with chlorine, bromine, and iodine, and forming:
Se8 (s) + 16 Cl2 (g) → 8 SeCl4 (s) (Selenium (IV) Chloride)
Se8 (s) + 16 Br2 (g) → 8 SeBr4 (s) (Selenium (IV) Bromide)
Se8 (s) + 16 I2 (g) → 8 SeI4 (s) (Selenium (IV) Iodide)
Selenium does not react with dilute non-oxidizing acids.
Selene(VI) as selenates is oxidized to selene(VI) by strong hydrochloric acid:
SeO42- (aq) + 2 H+ (aq) + 2 Cl– (aq) ⇌ H2SeO3 (aq) + Cl2 (aq) + H2O (l)
Selenous acid can also be made directly by oxidizing elemental selenium with nitric acid:
3 Se + 4 HNO3 + H2O → 3 H2SeO3 + 4 NO (stable trioxide)
Selenium trioxide is thermodynamically unstable and decomposes to the dioxide above 185 °C:
2 SeO3 → 2 SeO2 + O2 (ΔH = −54 kJ/mol)
Hydrogen sulfide reacts with aqueous selenous acid to produce selenium di-sulfide:
H2SeO3 + 2 H2S → SeS2 + 3 H2O
Selene(IV) as selenious acid is oxidized to selenic acid by permanganate.
5 H2SeO3 (aq) + 2 MnO4_ (aq) ⇌ 4 H+ (aq) + 5 SeO42- (aq) + H2O (l)
With highly electropositive metals, such as aluminium, selenides are prone to hydrolysis:
Al2Se3 + 3 H2O → Al2O3 + 3 H2Se
Selenium reacts with cyanides to yield selenocyanates:
8 KCN + Se8 → 8 KSeCN
Selenium History
Naming: After Selene, Greek goddess of the moon
Discovery and First isolation: Jöns Jakob Berzelius and Johann Gottlieb Gahn (1817)
Selenium Uses
ASelenium has good photovoltaic (converts light to electricity) and photoconductive (electrical resistance decreases with increased illumination) properties, and it is used extensively in electronics, such as photocells, photocopiers (Xerography for reproducing and copying documents, letters, etc), light meters and solar cells.
The largest use of selenium is in the glass industry, as an additive to glass for decolourise glass (remove colour from glass), and give a deep red colour to glasses and enamels.
It can also be used to reduce the transmission of sunlight in architectural glass, giving it a bronze tint.
It is used to make pigments for ceramics, paint and plastics.

Selenium taking about 15% in sodium selenite (Na2SeO3) for animal feeds and food supplements.
ASelenium is used in metal alloys such as the lead plates used in storage batteries and in rectifiers to convert AC current in DC current.
It is used as an additive to make stainless steel, and to improve the abrasion resistance in vulcanized rubbers.
ASelenium is toxic to the scalp fungus that causes dandruff, so it is used in some anti-dandruff shampoos.
Biological role
ASelenium has been said to be practically nontoxic and is considered to be an essential trace element for some species, including humans.
Human bodies contain about 14 milligrams, and every cell in a human body contains more than a million selenium atoms.
Too little selenium can cause health problems, but too much is also dangerous.
In excess, it is carcinogenic and teratogenic (disturbs the development of an embryo or foetus).
Hydrogen selenide (H2Se) and other selenium compounds are extremely toxic, and resemble arsenic in their physiological reactions.
Hydrogen selenide at a concentration of 1.5 ppm is intolerable to man.
Abundance of Selenium
Uncombined selenium is found in a few rare minerals, some of which can have as much as 30% selenium, and they are generally occur together with sulfides of metals such as copper, zinc and lead.
ASelenium is obtained from the anode muds, which produced during the electrolytic refining of copper.
These muds are either roasted with sodium carbonate (Na2Co3) or sulfuric acid (H2SO4), or smelted with sodium carbonate to release the selenium.
Annual world wide production is around 2000 tons.
30×10-7% (In Universe)
0.0013% (In Meteorites)
5×10-6% (In Earth’s Crust)
4.5×10-8% (In Oceans)
5×10-6% (In Humans)
World’s Top 3 producers of Selenium
1) Japan
2) Germany
3) Belgium
World’s Top 3 Reserve holders of Selenium
1) Russia
2) Chile
3) Peru
#Selenium


