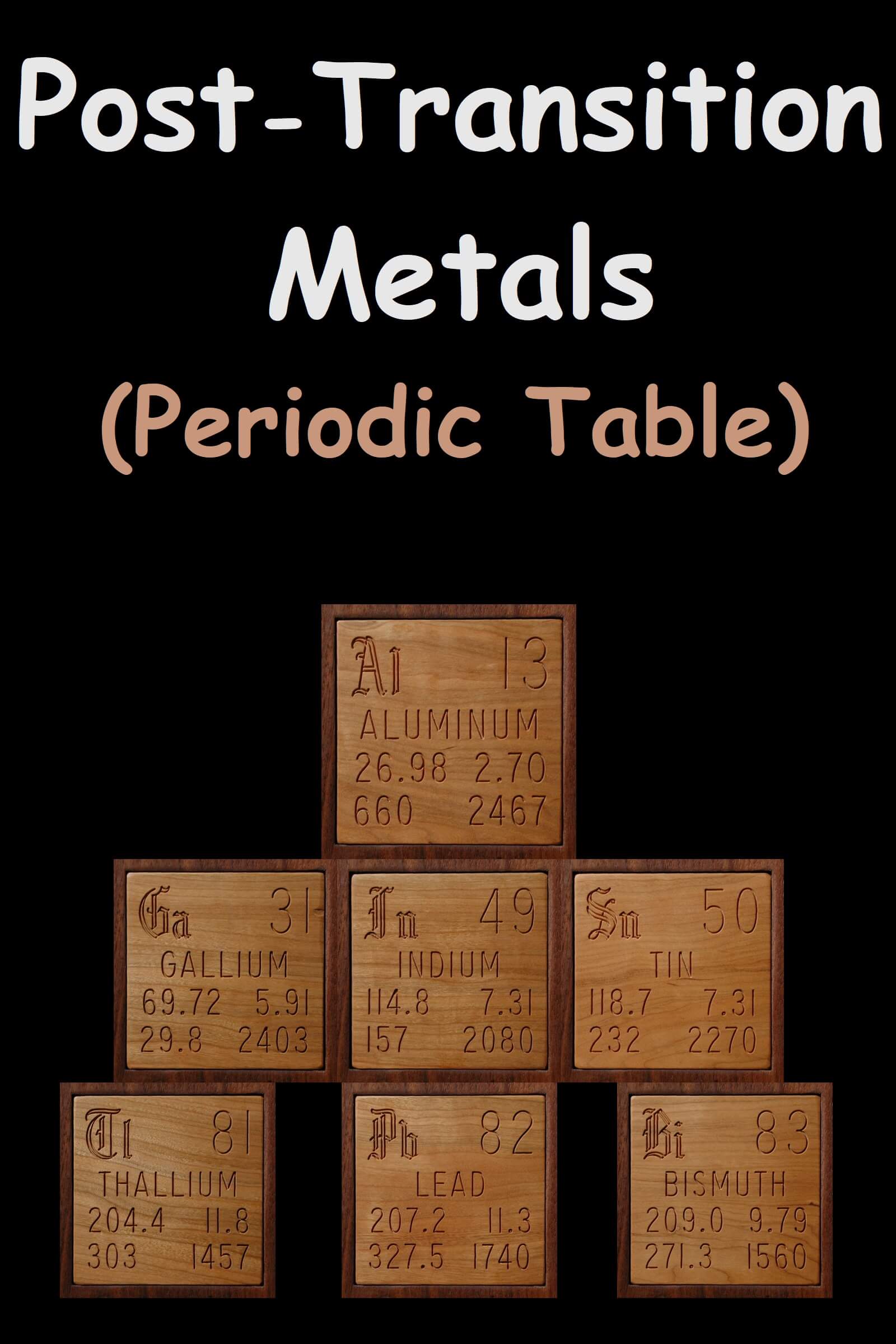
Post Transition Metals On The Periodic Table (Elements)
CONTENT INDEX
Introduction (Overview)
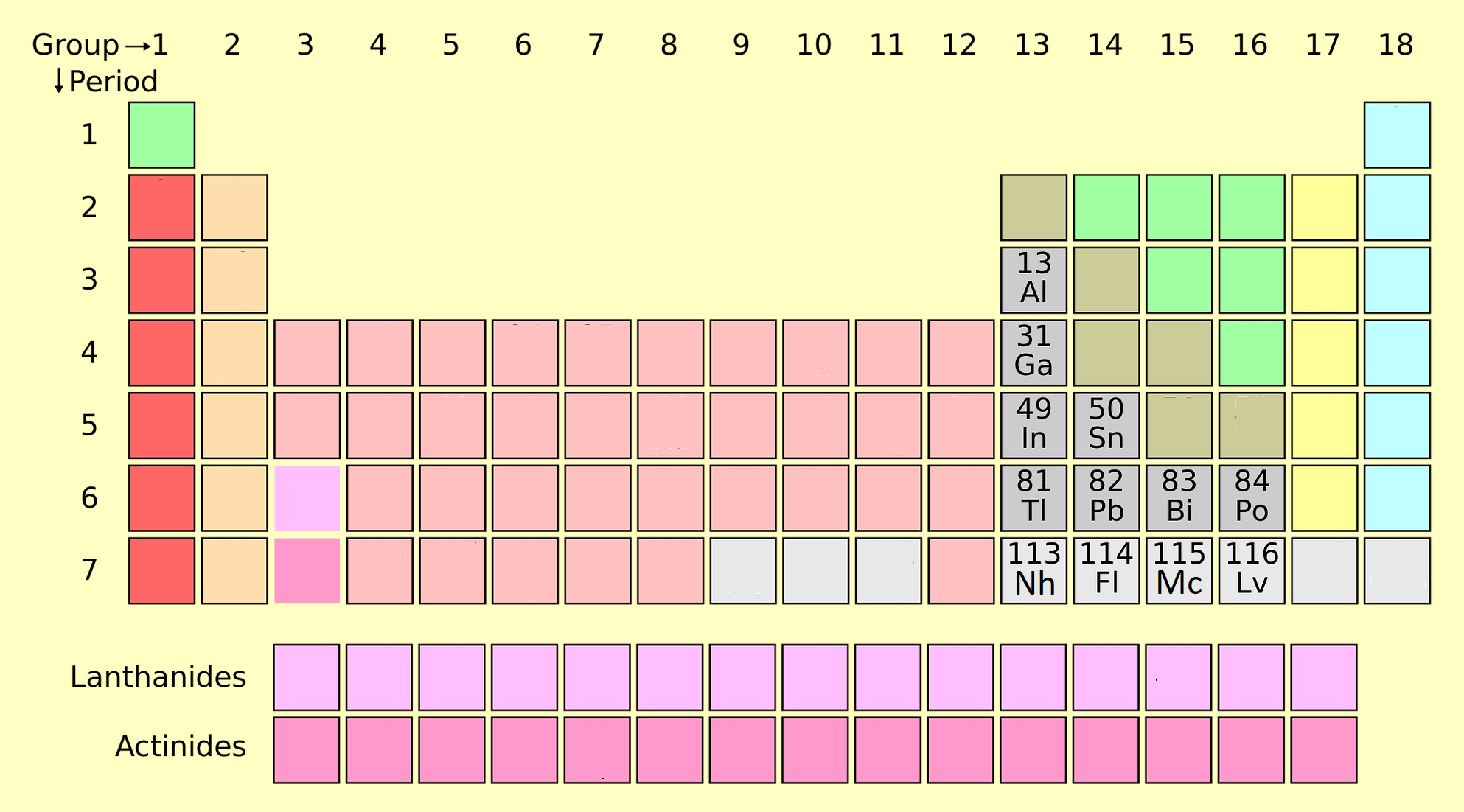
Post transition metals are a set of metallic elements in the periodic table. It is located between the transition metals on the left and the metalloids on the right, depending on where these neighboring groups should begin at the end. There are at least five competing proposals for the elements to be included. All proposal includes Gallium (Ga), Indium (In), Tin (Sn), Thallium (Th), Lead (Pb) and Bismuth (Bi). Other names for this group are B sub group metal under p block metals. Elements 112 and 118 are post-transition metals that have not been synthesized in sufficient quantities to study their true physical and chemical properties. Some other elements are also consider as post-transition elements like Aluminum (Al), Nihonium (Nh), Flerovium (Fl), Moscovium (Mo), Livermorium (Lv).
Several systems are used to classify elements belonging to metals according to transition metals. The most important of these are according to this definition:
- Groups 13 and higher, and periods 3 and higher only include metals that form relatively soft, electron-rich cations and contribute significantly to the bond. However, if this scheme is implemented very carefully, Al will be removed because it does not technically follow a d-block (and has an unfilled subshell (n-1) d and metals will be removed, although many of them are also soft. The ratio from electron-rich cations to undercharged shells (n-1) d) transitions and the problem is which elements should be classified as metal or metalloids.
- Metalloids and metals of the p block: This system has the advantage of highlighting the exciting and unique properties of metals and metalloids in the p-block, as well as its property continuity through the p-block. Hence it is used in the following sections. However, this metal has the disadvantages of removing metals such as Zn, Cd and Hg, which leads to an imbalance in many compounds in which the metal has the (n-1) d10.
- Metals following the transition elements in the forming of Ions with full (n-1) d valence, are sometimes combined with Al and p block metalloids. This definition adds Zn, Cd, and Hg (and sometimes Cu, Ag, and Au) as ions with electron-configured capacity (n-1) such as Zn2, Cd2, and Hg2 (Cu+, Ag+, and Au+) are formed.
Properties
Physical Properties of Post Transition Metals
- Post transition metals are soft and brittle.
- Poor mechanical strength.
- Have Lower boiling and melting points than of transition metals.
- Their crystal structures tend to show covalent or directional binding effects.
- They are generally more complex or less closely related than other metal elements.
- Ductile and malleable elements.
- They are good at thermal and electrical conductivity.
- They are also called poor metals.
Chemical Properties of Post Transition Metals
- They are characterized to varying degrees by covalent bonding tendencies.
- They do formation of anionic species such as aluminates, stannates, and bismuthates in the case of aluminum, tin, and bismuth, respectively.
- They can also form Zintl phases half metallic compounds formed between highly electropositive metals and moderately electronegative metals and metalloids.
- They show high electronegativity values corresponds to increasing nonmetallic character.
- Atomic radii contract ionization energies increase fewer electrons become available for metallic character.
Uses of post transition metals
- Aluminum (Al) is used to making in aero plane parts.
- Gallium (Ga) is used as a substitute in thermometer rather than mercury.
- Tin (Sn) is used to coat metal cans to prevent corrosion.
- Thallium (Th) was used as a rat poison and it is not used like that today because of its toxicity levels. Toxicity of this element is very high.
- Lead (Pb) is used to make lead acid batteries and is also dangerous.
- Bismuth (Bi) is used at fire tractors.
Here we mention some interesting facts which is related to post transition elements.
Some Interesting Facts related to post transition
- Zinc, cadmium, and mercury are included with the post-transition metals rather than the transition metals.
- Aluminum is the third most abundant element in the Earth’s crust after oxygen and silicon. Occasionally germanium and antimony are distributed as post-transition metals rather of metalloids.
- Gallium’s melting point is only slightly above room temperature and it can melt if held in the hand for some time.
- Bismuth is used in Pepto-Bismol, a medicine used to help upset stomach. Bismuth was formerly allowed to be the heaviest stable element, but lately it was discovered to be slightly radioactive.
- Indium is used in electronics including flat panel displays and touch defenses. The name thallium comes from the Greek word “thallos” which means” a green shoot or branch.” Thallium is largely poisonous and can beget death. One another symptom of thallium poisoning is loss of hair.
We have discussed above about the post transition metals and why they called as post transition metals or what are their physical and chemical properties. We have also discussed about metals and metalloids. So, now I hope you get all the information about post transition metals.