Transition Metals On The Periodic Table
CONTENT INDEX
Introduction (Overview)
- Elements in groups 3rd to 12th are d-block metals, often referred to as transition metals.
- Transitions metals that are in the middle of the periodic table.
- They have giant metallic structure, and high melting points.
- They can conduct electricity and are good conductors of heat as well.
- Because of their high melting points as well some of the metals can be useful for such scenarios where you need a large amount of heat that could be in jet agencies, you can use titanium for example.
- They also have some strengths as well, you can use iron to make steel.
- We are also going to look at electron configuration of the elements and the ions that they form. We are going to look at their chemical properties and physical properties. We will also discuss about some definitions of a transition metals and d block elements, because it is important that we need to know the difference between them. So, let’s study about it.
Transition metals
These metals form stable ion that has partially filled d-subshell are known as transition metal. This is different to d block elements. D-block elements are any element that has its outer most electrons in highest energy level and occupied d-orbital.
Transition metals List: Transition metals are Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), Zinc (Zn), Yttrium (Y), Zirconium (Zr), Niobium (Nb), Molybdenum (Mo), Technetium (Tc), Ruthenium (Ru), Rhodium (Rh), Palladium (Pd), Silver (Ag), Cadmium (Cd), Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium (Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au), Mercury (Hg), Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrium (Bh), Hassium (Hs), Meitnerium (Mt), Darmstadtium (Ds), Roentgenium (Rg), and Copernicium (Cn).
Transition Metal Properties
- Scandium and zinc are not transition metals. Because of Scandium’s stable ion is Sc3+ So, three electrons lose from scandium atom with left an ion that has an electron configuration with no electrons in the d-orbital.
- So, because of that is not partially filled, We can say that scandium is not a transition metal.
- Electron configuration of transition metals: They always have electrons in the 4s orbital and some of them fully filled orbital so, they have two electrons in there and some of them partially filled.
- 4s and 3d in the same row, so there’s no energy difference but the 4s and 3d orbitals are actually really close together in terms of energy and that’s why we’ve got the word transition.
- Electrons can move relatively easily between the 4s and the 3d orbitals.
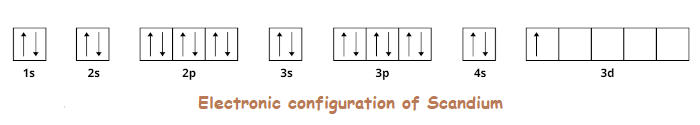
- These orbitals can change in terms of energy levels. So, let’s start with first element scandium (Sc);
- Scandium has 2 electrons in 4s orbital and 1 electron in 3d orbital (Incomplete d-orbitals). 1s22s22p63s23p63d14s2, we can write it as also; [Ar] 3d¹ 4s²
- This same thing goes on with Titanium (Ti), Vanadium(V), But it is little bit different for chromium (Cr) and copper (Cu).
- Electronic configuration of chromium is [Ar] 3d⁵ 4s¹, there’s half-filled d-orbital.
- Electron configuration of cu is [Ar] 3d¹⁰ 4s¹, In which fully filled d-orbital, that is highly stable.
- They form ions due to removal of electrons.
- Firstly, electrons are removed from the s orbital because of their higher energy levels.
- So, it is easy to remove, For example; Mn2+ = [Ar] 3d5.
Now look at the physical properties of transition metals.
Physical Properties of Transition Metals
- They are good conductors of electricity. So, they are used in electrical circuits.
- Transition metals are good conductors of thermal energy therefore they are used in radiators and also in cooking pots.
- They also have a high density and a high melting point.
- All transition metals are solid at room temperature except for mercury, Only mercury (Hg) is a liquid at room temperature. But still, it is a metal.
- Transition metals are stronger than alkali metals.
- They all are pure metals and they used in different industries like Ni, Co, cd, w etc.
- Transition metals are used for alloy formation. For example- steel, brass, bronze etc.
- They are interstitial compounds.
Chemical Properties of Transition Metals
- They are used I industry in particularly for things like catalyst and paint etc.
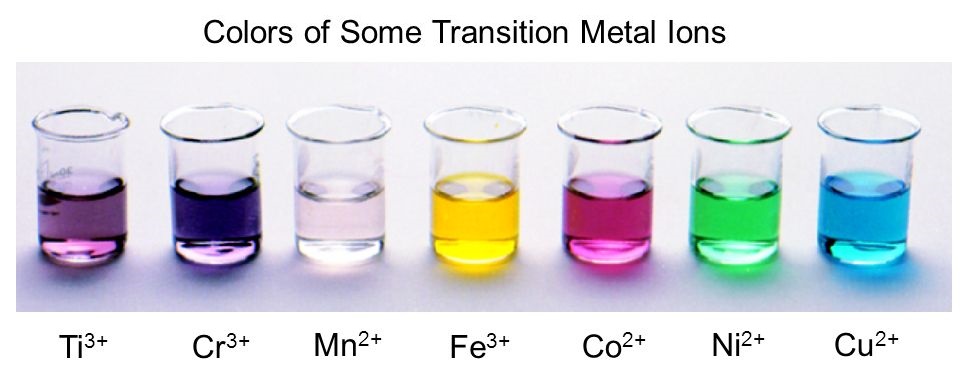
- All transition metals have a feature of color. For example- fe2+ – green color, fe3+– reddish orange color.
- Lot of these transition metals are colorful metals.
- They form colored metal ions and complexes.
- They are really good catalyst. For example- we can use iron as a catalyst in the Haber process.
- They show variable oxidation states. For example- fe2+ and fe3+ are same atoms but show different oxidation states.
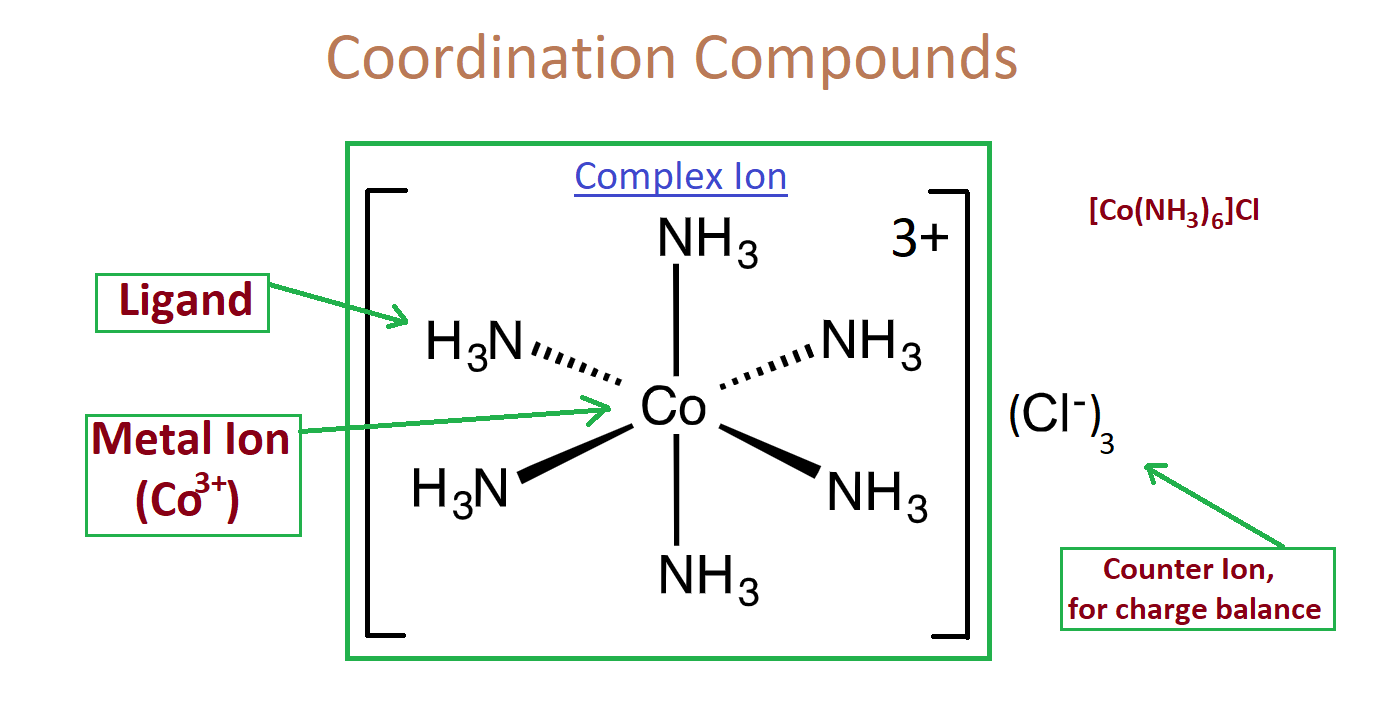
- Transition metals have a unique quality to form large complexes. So, for example- Cu2+, if you dissolve copper in water and it can form copper and 6 molecules of H2O.
Cu2+ + 6H2O → [ Cu (H2O)6 ]2+
- They are Para magnetism.
- They are paramagnetic from starting elements up to middle, After that pairing is start and they will turn into diamagnetic.
- Zn, cd, Hg are known as non-typical transition elements due to the presence of fully filled d- orbitals.
- Inner Transition Elements: inner transition elements are those whose electrons is filled in f orbitals. They are found in lanthanide and actinide series. They are present in last of the series. They are also known as f block elements.
- Outer Transition Metals: All d block elements are called as outer transition metals.
- Binding force of the elements is decreases as we move left to the right.
- Their reactivity is lower than alkali metals and alkaline earth metals.
Uses of Transition Elements
- Transition metals are often used as catalyst. Catalyst alters the rate of a reaction by lowering activation energy.
- Due to their physical properties, transition metals are Important metals in engineering.
- Many dyes and paints contain transition metal compounds.
- Iron (Fe) is used in making building, ships and cars.
- Copper (Cu) is generally used in making electricity cables.
- Gold (Au) is used in jewelries. It does not corrode in air or water.
- Tungsten (W) is used in making filaments in electric lights.
- Manganese (Mn) is used for harden steel.
- Nickel (Ni) is used as a catalyst or in coins.
- Silver (Ag) is used in jewelry, It also does not corrode in the presence air and water.
We have discussed above about what are the transition metals and their physical and chemical properties or their uses. And, what’s their role in our daily life. So, Now I hope you get all the information about them.