46 Pd (Palladium)

Palladium is a lustrous silvery-white metal.
it does not tarnish in air (corrosion resistant).
It is attacked by hot acids, and dissolves in Aqua regia (mixture of nitric acid and hydrochloric acid).
At room temperature, the metal has the property of absorbing hydrogen up to 900 times of its own volume, and possibly forming Pd2H.
Like gold, palladium can be beaten into leaf as thin as 1/250,000 in.

Identity
CAS Number: CAS7440-05-3
CID Number: CID23938
DOT Hazard Class: 4.2
DOT Number: 3200
RTECS Number: RTECSRT3480500
CONTENT INDEX
Basic Properties of Palladium
Pronunciation: Pa-Lay-dee-am
Appearance: Silvery white
Mass Number: 106
Standard Atomic weight: 106.42 g/mol
Atomic number (Z): 46
Electrons: 46
Protons: 46
Neutrons: 60
Period: 5
Group: 10
Block: d
Element category: Transition metal
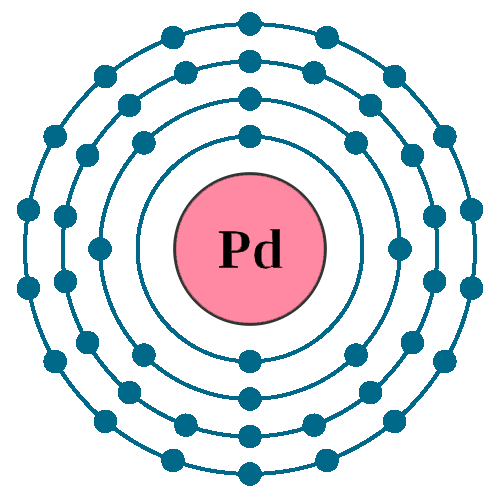
Electrons per shell: K2, L8, M18, N18
Electron configuration: 1s22s22p63s23p63d104s24p64d10
Thermal Properties of Palladium
Phase: Solid
Melting point: 1828.05 K (1554.9 oC, 2830.82 oF)
Boiling point: 3236 K (2963 oC, 5365 oF)
Debye temperature: 274 K (0.85 oC, 33.53 oF)
Fusion heat: 16.74 kJ/mol
Vaporization heat: 358 kJ/mol
Specific heat: 240 J/(kg K)
Molar heat capacity: 25.98 J/(mol.K)
Thermal expansion: 11.8 μm/(m∙K)
Thermal conductivity: 71.8 W/(m∙K)
Electrical properties of Palladium
Electrical conductivity: 10×106 S/m
A Electrical resistivity: 105.4 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Palladium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +567.4×10-6 cm3/mol
Volume magnetic susceptibility: 0.0007899
Mass magnetic susceptibility: 65.7×10-9 m3/kg
Molar magnetic susceptibility: 6.992×10-9 m3/mol
Physical Properties of Palladium
Density: 12.023 g/cm3 (In solid) 10.38 g/cm3 (In Liquid at M.P)
Molar volume: 0.000008.851 m3/mol
Young’s modulus: 121 GPa
Shear modulus: 44 GPa
Mohs Hardness: 4.75
Bulk modulus: 180 GPa
Poisson ratio: 0.39
Vicker hardness: 400-600 MPa
Brinell hardness: 320-610 MPa
Sound Speed: 3070 m/s
Atomic Properties of Palladium
Oxidation states: 0, +1, +2, +3, +4
Valence Electrons: 4d10
Ion charge: Pd2+ Pd4+
The ionization potential of an atom: 8.3
Ionization energies: 1st: 804.4 kJ.mol 2nd: 1870 kJ/mol 3rd: 3177 kJ/mol
Ionic radius: 86 pm
Atomic radius: 137 pm
Van der Waals: 163 pm
Covalent radius: 139±6 pm
Filling Orbital: 4d10
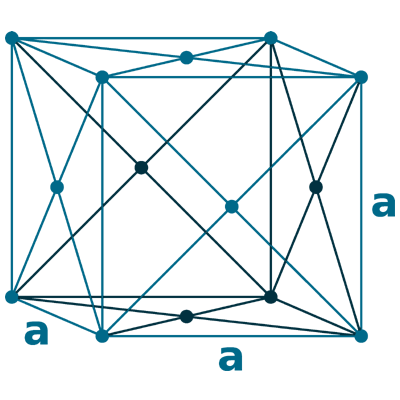
Crystal structure: Face-centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 389.07, 389.07, 389.07 pm
Grid parameters: 3.890 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Palladium
Electronegativity: pauling scale: 2.20
Valence: +4
Electron affinity: 53.7 kJ/mol
Nuclear Properties of Palladium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 6.9
Neutron Mass Absorption: 0.0023
Isotopes: 100Pd 102Pd 103Pd 104Pd 105Pd 106Pd 107Pd 108Pd 110Pd
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 100Pd | Syn | – | 3.63 d |
| 102Pd | 1.02 | 101.906 | Stable |
| 103Pd | Syn | – | 16.991 d |
| 104Pd | 11.14 | 103.904 | Stable |
| 105Pd | 22.33 | 104.905 | Stable |
| 106Pd | 27.33 | 105.903 | Stable |
| 107Pd | Trace | – | 6.5×106 y |
| 108Pd | 26.46 | 107.904 | Stable |
| 110Pd | 11.72 | 109.905 | Stable |
Chemical Reactions
Palladium metal react with oxygen by heating.
2 Pd (s) + O2 (g) → PdO (s) [black] (palladium (II) oxide)
The metal reacts with halogens:
In reaction with Fluorine gas, forming a mixture of Pd(II) and Pd(IV), Not a Palladium (III) fluoride
2 Pd (s) + 3 F2 (g) → [Pd(II)][Pd(IV)F6] (s)
Pd (s) + Cl2 (g) → PdCl2 (s) [red] (Pd(II)chloride)
PdCl2 reacts with ligands (L) to give square planar complexes of the type PdCl2L2
PdCl2 + 2 L → PdCl2L2 (L = PhCN, PPh3, NH3, etc)
Reduction of a mixture of PdCl2(PPh3)2 and PPh3 gives tetrakis (triphenylphosphine) palladium(0):
2 PdCl2(PPh3)2 + 4 PPh3 + 5 N2H4 → 2 Pd(PPh3)4 + N2 + 4 N2H5+Cl−
Pd (s) + Br2 (g) → PdBr2 (s) [red-black] (Pd (II) bromide)
Palladium History
Naming: After asteroid Pallas, itself named after Pallas Athena (Greek: Pallas goddess of wisdom)
Discovery and First isolation: William Hyde Wollaston (1802)
Palladium Uses
Palladium is a good catalyst and is used for hydrogenation and dehydrogenation reactions, where Hydrogen easily diffuses through heated palladium, that provides a way of separating and purifying the gas.
It is mostly used in catalytic converters for cars.
It is also used in jewellery, in making surgical instruments, and some dental fillings and crowns.
White gold is an alloy of gold that has been decolorized by the addition of palladium.
Palladium is used in the electronics industry as in ceramic capacitors, in laptop, computers and mobile phones.

Biological role: It is not-toxic.
Abundance of Palladium
Palladium has been found uncombined in nature, but mostly found in sulfide minerals such as braggite.
It is extracted commercially as a by-product of Nickel refining, also extracted as a by-product of copper and zinc refining.
Palladium’s separation from the platinum metals depends upon the type of ore in which it is found.
Annual world wide production is around 24 tons.
2×10-7% (In Universe)
6.5×10-5% (In Meteorites)
3×10-7% (In Sun)
6.3×10-7% (In Earth’s Crust)

World’s Top 3 producers of Palladium
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Palladium
1) South Africa
2) Russia
3) USA
#palladium


