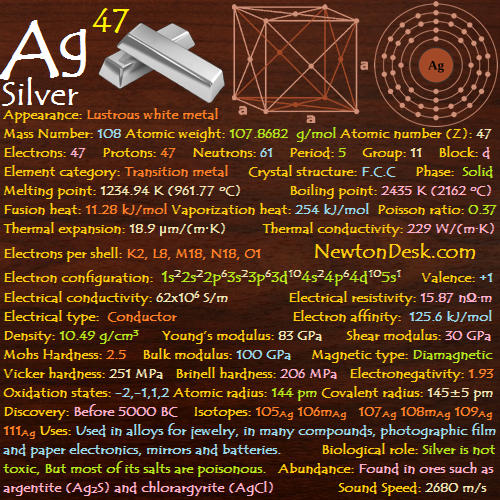
47 Ag (Silver)
Pure silver has a brilliant white metallic luster, very ductile, malleable, and little harder than gold.
It has the highest electrical conductivity from all other metals, But due to High purchasing cost, It is not widely used for electrical purposes.
It is not a chemically active metal, but attacked by nitric acid and hot concentrated sulfuric acid (H2SO4).
It is stable in pure air and water, but tarnishes when contact to ozone, hydrogen sulfide or air containing sulfur, forming silver sulfide.
That’s why silver objects need regular cleaning.

Identity
CAS Number: CAS7440-22-4
CID Number: CID23954
RTECS Number: RTECSVW3500000
CONTENT INDEX
Basic Properties of Silver
Appearance: Lustrous white metal
Mass Number: 108
Standard Atomic weight: 107.8682 g/mol
Atomic number (Z): 47
Electrons: 47
Protons: 47
Neutrons: 61
Period: 5
Group: 11
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N18, O1
Electron configuration: 1s22s22p63s23p63d104s24p64d105s1

Thermal Properties of Silver
Phase: Solid
Melting point: 1234.94 K (961.77 oC, 1763.2 oF)
Boiling point: 2435 K (2162 oC, 3924 oF)
Debye temperature: 225 K (-48.15 oC, -54.67 oF)
Fusion heat: 11.28 kJ/mol
Vaporization heat: 254 kJ/mol
Specific heat: 235 J/(kg K)
Molar heat capacity: 25.350 J/(mol.K)
Thermal expansion: 18.9 μm/(m∙K)
Thermal conductivity: 229 W/(m∙K)
Electrical properties of Silver
Electrical conductivity: 62×106 S/m
A Electrical resistivity: 15.87 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Siver
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -19.5×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000238
Mass magnetic susceptibility: -2.27×10-9 m3/kg
Molar magnetic susceptibility: -2.45×10-9 m3/mol
Physical Properties of Silver
Density: 10.49 g/cm3 (In solid) 9.320 g/cm3 (In Liquid at M.P)
Molar volume: 0.000010283 m3/mol
Young’s modulus: 83 GPa
Shear modulus: 30 GPa
Mohs Hardness: 2.5
Bulk modulus: 100 GPa
Poisson ratio: 0.37
Vicker hardness: 251 MPa
Brinell hardness: 206-250 MPa
Sound Speed: 2680 m/s
Atomic Properties of Silver
Oxidation states: -2, -1, 1, 2, 3
Valence Electrons: 4d10 5s1
Ion charge: Ag+
The ionization potential of an atom: 7.54
Ionization energies: 1st: 731 kJ.mol 2nd: 2070 kJ/mol 3rd: 3361 kJ/mol
Ionic radius: 126 pm
Atomic radius: 144 pm
Van der Waals: 172 pm
Covalent radius: 145±5 pm
Filling Orbital: 4d10
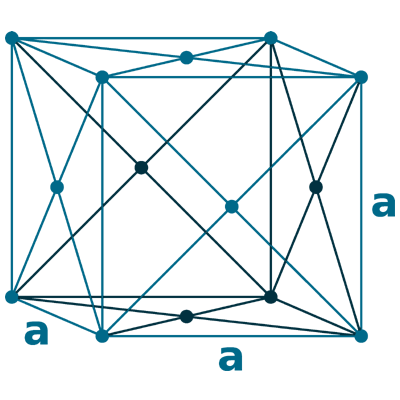
Crystal structure: Face-centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 408.53, 408.53, 408.53 pm
Grid parameters: 4.085 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Silver
Electronegativity: pauling scale: 1.93
Valence: +1
Electron affinity: 125.6 kJ/mol
Nuclear Properties of Silver
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 63.6
Neutron Mass Absorption: 0.02
Isotopes: 105Ag 106mAg 107Ag 108mAg 109Ag 111Ag
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 105Ag | Syn | – | 41.2 d |
| 106mAg | Syn | – | 8.28 d |
| 107Ag | 51.84 | 51.839 | Stable |
| 108mAg | Syn | – | 418 y |
| 109Ag | 48.16 | 48.161 | Stable |
| 111Ag | Syn | – | 7.45 d |
Chemical Reactions
Silver metal is stable in clean air under normal conditions, as well as not react with clean water.
The metal reacts with all Halogens:
Ag (s) + F2 (g) → AgF2 (s) [Brown] (Silver (ll) difluoride)
Ag+ is precipitated by halides, that can be dissolved again in concentrated halides.
Ag+ (aq) + Cl– (aq) → AgCl (s) [white]
AgCl (s) + Cl– (aq) → [AgCl2]– (aq) + Cl– (aq) → [AgCl3]2- (aq) + Cl– (aq) → [AgCl4]3- (aq)
Ag+ (aq) + Br– (aq) → AgBr (s) [Light yellow]
AgBr (s) + Br– (aq) → [AgBr2]– (aq) + Br– (aq) → [AgBr3]2- (aq) + Br– (aq) → [AgBr4]3- (aq)
Ag+ (aq) + I– (aq) → AgI (s) [Yellow]
AgI (s) + I– (aq) → [AgI2]– (aq) + I– (aq) → [AgI3]2- (aq) + I– (aq) → [AgI4]3- (aq)
Metal dissolves in dilute nitric acid:
3 Ag (s) + 4 HNO3 (aq) → AgNo3 (aq) + NO (g) + 2 H2O (I)
silver azide, AgN3, formed by reaction of aqueous solution of silver nitrate with sodium azide.
AgNo3 (aq) + NaN3 (aq) → AgN3 (s) + NaNo3 (aq)
In reaction silver azide decomposes explosively and releasing nitrogen gas:
2 AgN3 (s) → 3 N2 (g) + 2 Ag (s)
The liquid lead oxide is removed or absorbed by capillary action into the hearth linings.
Ag(s) + 2Pb(s) + O2(g) → 2PbO(absorbed) + Ag(l) (Production)
Silver History
Naming: Latin argentum (silver). Silver from Anglo-Saxon seolfor for silver.
Discovery: Before 5000 BC
Silver Uses
Sterling silver (contains 92.5% silver and remain copper or other metal) is used for jewelry, silverware, etc.. where appearance is important.
Silver is used to make mirrors, Because it is the best reflector of visible light known, but is rapidly tarnished and loses much of its reflectance with time.
It is a poor reflector of ultraviolet.
It is also used in dental alloys, making solder and brazing alloys, electrical contacts and and high capacity silver-zinc (Ag-Zn) and silver-cadmium (Ag-Cd) batteries.
A Silver paints are used for making printed circuits.
A Silver fulminate is a powerful explosive, which is sometimes formed during the silvering process.
The Silver iodide is used in seeding clouds to produce Artificial rain.
Silver nitrate or lunar caustic is the most important silver compound, which is used extensively in photography, about 30% of the U.S. industrial consumption going into this application (producing high-quality images and protecting against illegal copying).
The Silver chloride has interesting optical properties as It darkens in bright sunlight and becomes transparent in low sunlight.
Due to antibacterial properties, silver nanoparticles are used in clothing to prevent bacteria from digesting sweat and forming unpleasant odors.
Biological role of Silver
Silver itself is not toxic, But most of its salts are poisonous.
Exposure to silver in air should not exceed 0.01 mg/m3.
Silver compounds can be absorbed (by Chronic ingestion or inhalation) in the circulatory system and reduced silver deposited in the various tissues of the body.
Result condition known as Argyria (the skin and mucous membranes turns purple or purple-grey).
Silver has antibacterial properties and can kill lower organisms quite effectively without harm to higher animals.
Abundance of Silver
Silver occurs uncombined, and in ores such as argentite (Ag2S) and chlorargyrite (horn silver, AgCl).
It is Chiefly extracted from lead-zinc, copper, gold and copper-nickel ores.
The metal also recovered during the electrolytic refining of copper.
Commercial fine silver contains at least 99.9% silver.
Purities of 99.999+% are available commercially.
Annual world wide production is around 26800 tons.
6×10-8% (In Universe)
1.4×10-5% (In Meteorites)
1×10-7% (In Sun)
7.9×10-6% (In Earth’s Crust)
1×10-8% (In Oceans)


World’s Top 3 producers of Silver
1) Mexico
2) Peru
3) China
World’s Top 3 Reserve holders of Silver
1) Peru
2) Poland: chile
#Silver

Thanks, it is very informative