48 Cd (Cadmium)
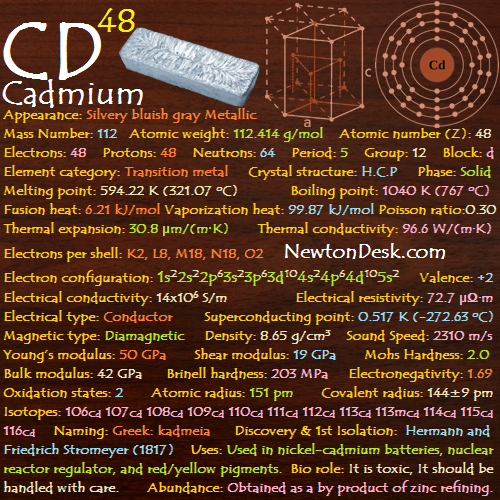
Cadmium is a soft, lustrous, bluish-silvery-white metal which can be easily cut with a knife.
It is tarnishes in air, and soluble in acids but not in alkalis.
It is similar in many respects to zinc.

Identity
CAS Number: CAS7440-43-9
CID Number: CID23973
DOT Hazard Class: 6.1
DOT Number: 2570
RTECS Number: RTECSEU9800000
CONTENT INDEX
Basic Properties of Cadmium
Pronunciation: Kad-mee-am
Appearance: Silvery bluish gray Metallic
Mass Number: 112
Standard Atomic weight: 112.414 g/mol
Atomic number (Z): 48
Electrons: 48
Protons: 48
Neutrons: 64
Period: 5
Group: 12
Block: d
Element category: Transition metal
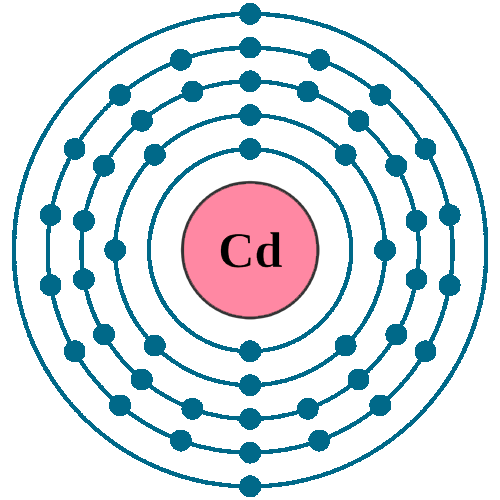
Electrons per shell: K2, L8, M18, N18, O2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s2
Thermal Properties of Cadmium
Phase: Solid
Melting point: 594.22 K (321.07 oC, 609.93 oF)
Boiling point: 1040 K (767 oC, 1413 oF)
Debye temperature: 209 K (-64.15 oC, -83.47 oF)
Fusion heat: 6.21 kJ/mol
Vaporization heat: 99.87 kJ/mol
Specific heat: 230 J/(kg K)
Molar heat capacity: 26.020 J/(mol.K)
Thermal expansion: 30.8 μm/(m∙K)
Thermal conductivity: 96.6 W/(m∙K)
Electrical properties of Cadmium
Electrical conductivity: 14×106 S/m
A Electrical resistivity: 72.7 μΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.517 K (-272.63 oC, -458.73 oF)
Magnetic Properties of Cadmium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -19.8×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000199
Mass magnetic susceptibility: -2.3×10-9 m3/kg
Molar magnetic susceptibility: -0.26×10-9 m3/mol
Physical Properties of Cadmium
Density: 8.65 g/cm3 (In solid) 7.996 g/cm3 (In Liquid)
Molar volume: 0.000012996 m3/mol
Young’s modulus: 50 GPa
Shear modulus: 19 GPa
Mohs Hardness: 2.0
Bulk modulus: 42 GPa
Poisson ratio: 0.30
Brinell hardness: 203-220 MPa
Sound Speed: 2310 m/s
Atomic Properties of Cadmium
Oxidation states: 2
Valence Electrons: 5s2
Ion charge: Cd2+
The ionization potential of an atom: 8.95
Ionization energies: 1st: 867.8 kJ.mol 2nd: 1631 kJ/mol 3rd: 3616 kJ/mol
Ionic radius: 97 pm
Atomic radius: empirical: 151 pm
Van der Waals: 158 pm
Covalent radius: 144±9 pm
Filling Orbital: 4d10
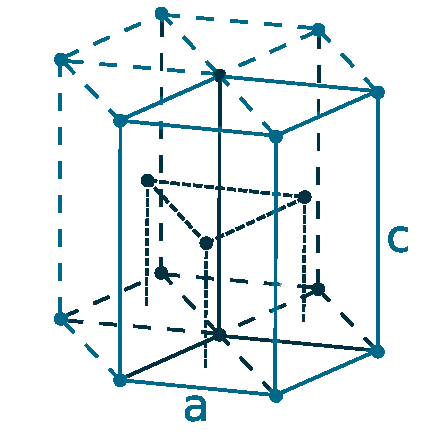
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 297.94, 297.94, 561.8 pm
Grid parameters: a=2.979 Å c=5.618 Å
Attitude c/a: 1.886
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Cadmium
Electronegativity: pauling scale: 1.69
Valence: +2
Electron affinity: 0 kJ/mol
Nuclear Properties of Cadmium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 2450
Neutron Mass Absorption: 1.4
Isotopes: 106Cd 107Cd 108Cd 109Cd 110Cd 111Cd 112Cd 113Cd 113mCd 114Cd 115Cd 116Cd
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 106Cd | 1.25 | 105.906 | Stable |
| 107Cd | Syn | – | 6.5 h |
| 108Cd | 0.89 | 107.904 | Stable |
| 109Cd | Syn | – | 462.6 d |
| 110Cd | 12.47 | 109.903 | Stable |
| 111Cd | 12.80 | 110.904 | Stable |
| 112Cd | 24.11 | 111.903 | Stable |
| 113Cd | 12.23 | 112.904 | 7.7×1015 |
| 113mCd | Syn | – | 14.1 y |
| 114Cd | 28.75 | 113.903 | Stable |
| 115Cd | Syn | – | 53.46 h |
| 116Cd | 7.51 | 115.905 | 3.1×1019 y |
Chemical Reactions
Cadmium doesn’t react with air, under normal pressure and temperature.
At 300 oC Cd forms a thin brown gas-permeable coating.
The metal burns(above the sublimation temperature of CdO, 1380 OC) in air to form Cadmium (II) oxide.
2 Cd (s) + O2 (g) → 2 CdO (s)
Cadmium reacts with water (H2O) and Forming H2 (g) (At Above 400 oC):
Cd (s) + H2O (g) → CdO (s) [Reddish-brown]
Cd reacts very slowly with distilled water.
The metal reacts with all Halogens:
Cd (s) + F2 (g) → CdF2 (s)
Cd (s) + Cl2 (aq) → Cd2+ (aq) + 2 Cl– (aq)
Cd (s) + Br2 (aq) → Cd2+ (aq) + 2 Br– (aq)
Cd (s) + Br2 (g) → CdBr2 (s) [pale yellow]
Cd (s) reacts with I2 (aqueous), But not react I2 (gas).
Cd (g) reacts with I2 and forming Cdl2.
Cd (s) + I2 (aq) → Cd2+ (aq) + 2 I– (aq)
Cd (g) + I2 (g) → Cdl2 (g)
2 Cd (g) + I2 (g) → 2 Cdl (g) [At high temperature and Pressure (equivalent a steel bomb)]
Dissolves slowly in dilute sulfuric acid to form Cd (ll) ion and hydrogen:
Cd (s) + H2SO4 (aq) → Cd2+ (aq) + SO42– (aq) + H2 (g)↑
Cd dissolves in dilute hot nitric acid, and forming nitrogen monoxide:
3 Cd (s) + 2 NO3- (aq) + 8 H+ (aq) → Cd2+ (aq) + 2 NO (g) + H2O (l)
Cadmium History
Naming: Greek: kadmeia (ancient name for calamine (ZnCO3)); Latin: cadmia.
Discovery and First Isolation: Hermann and Friedrich Stromeyer (1817)
Named By: Friedrich Stromeyer (1817)
Cadmium Uses
It is Poison and cause birth defects and cancer, So it has limited use.
About 80% of cadmium is used in rechargeable Nickel-Cadmium (Ni-Cd) batteries, But now a days it is replaced with Nickel metal hydride batteries.
A Cadmium is extensively (about 60%) used in electroplate steel (film of cadmium only 0.05 mm thick can provide complete protection against the corrosion). It is still used today to protect critical components of Aeroplanes and oil platforms.
A Cadmium has the ability to absorb neutrons, so it is used in rods in nuclear reactors to control atomic fission.
Cadmium compounds were used in black and white phosphors for B&W TV tubes and in blue and green phosphors for color TV tubes.
Other uses of cadmium are mainly for coatings and plating, pigments (yellow, orange and red), and as stabilizers for plastics.
Biological role
Cadmium is toxic, carcinogenic and teratogenic (disturbs the development of an embryo or foetus), So It should be handled with care.
On average we take in as little as 0.05 milligrams per day and It store in the body on average about about 50 milligrams.
Abundance of Cadmium
Cadmium mostly occurs in small quantities associated with zinc ores, such as sphalerite (ZnS).
Greenockite (Cadmium sulfide, CdS) contains significant quantities of cadmium.
Almost all cadmium is obtained as a by-product in the treatment of zinc, copper, and lead ores.
Annual world wide production is around 13000 tons.
5×10-7% (In Universe)
1.7×10-5% (In Meteorites)
1×10-7% (In Sun)
0.0008% (In Earth’s Crust)
4.5×10-11% (In Oceans)


World’s Top 3 producers of Cadmium
1) China
2) Republic of Korea
3) Japan
World’s Top 3 Reserve holders of Cadmium
1) India
2) China
3) Australia
#cadmium