45 Rh (Rhodium)

Rhodium is an element of Platinum group metals (PGM) together with ruthenium, palladium, osmium, iridium, and platinum.
Rhodium is the rarest of all non-radioactive metals.
Rhodium is a lustrous and silvery white and at red heat slowly changes in air to the sesquioxide(an oxide containing three atoms of oxygen with two atoms of another element eg: Al2O3 , La2O3).
At higher temperatures It turns black to the element.
It is unaffected by air and water up to 600 oC, and insoluble in most acids, including Aqua regia (mixture of nitric acid and hydrochloric acid).
But it dissolved in hot concentrated sulfuric acid (H2SO4) and it is attacked by molten alkalis.
It has a higher melting point and lower density than platinum, and it is highly reflective, hard, and durable.


Identity
CAS Number: CAS7440-16-6
CID Number: CID23948
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSVI9069000
CONTENT INDEX
Basic Properties of Rhodium
Pronunciation: Roh-dee-am
Appearance: Silvery white Metallic
Mass Number: 103
Standard Atomic weight: 102.905 g/mol
Atomic number (Z): 45
Electrons: 45
Protons: 45
Neutrons: 58
Period: 5
Group: 9
Block: d
Element category: Transition metal
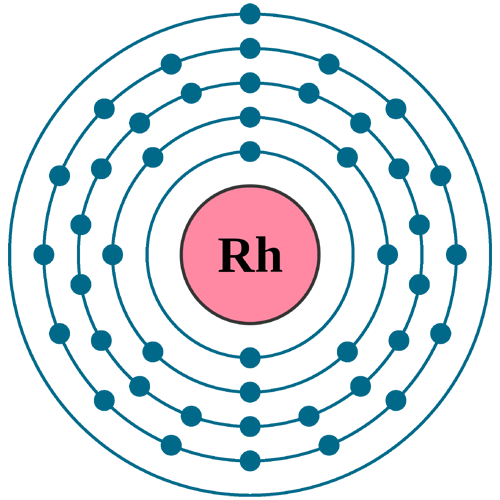
Electrons per shell: K2, L8, M18, N16, O1
Electron configuration: 1s22s22p63s23p63d104s24p64d85s1
Thermal Properties of Rhodium
Phase: Solid
Melting point: 2237 K (1964 oC, 3567 oF)
Boiling point: 3968 K (3695 oC, 6683 oF)
Debye temperature: 480 K (206.85 oC, 404.33 oF)
Fusion heat: 26.59 kJ/mol
Vaporization heat: 493 kJ/mol
Specific heat: 240 J/(kg K)
Molar heat capacity: 24.98 J/(mol.K)
Thermal expansion: 8.2 μm/(m∙K)
Thermal conductivity: 150 W/(m∙K)
Electrical properties of Rhodium
Electrical conductivity: 23×106 S/m
A Electrical resistivity: 43.3 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Rhodium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +111×10-6 cm3/mol
Volume magnetic susceptibility: 0.0001693
Mass magnetic susceptibility: 13.6×10-9 m3/kg
Molar magnetic susceptibility: 1.4×10-9 m3/mol
Physical Properties of Rhodium
Density: 12.41 g/cm3 (In solid) 10.7 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000082655 m3/mol
Young’s modulus: 380 GPa
Shear modulus: 150 GPa
Mohs Hardness: 6.0
Bulk modulus: 275 GPa
Poisson ratio: 0.26
Vicker hardness: 1100-8000 MPa
Brinell hardness: 980-1350 MPa
Sound Speed: 4700 m/s
Atomic Properties of Rhodium
Oxidation states: 6, 5, 4, 3, 2, 1, -1, -3
Valence Electrons: 4d8 5s1
Ion charge: Rh3+
The ionization potential of an atom: 7.7
Ionization energies: 1st: 719.7 kJ.mol 2nd: 1740 kJ/mol 3rd: 2997 kJ/mol
Ionic radius: 68 pm
Atomic radius: empirical: 134 pm
Van der Waals: 195 pm
Covalent radius: 142±7 pm
Filling Orbital: 4d8
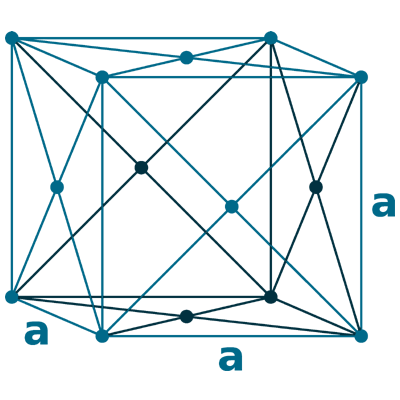
Crystal structure: Face-Centered Cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 380.34, 380.34, 380.34 pm
Grid parameters: a=3.803 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Rhodium
Electronegativity: pauling scale: 2.28
Valence: +6
Electron affinity: 109.7 kJ/mol
Nuclear Properties of Rhodium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4F9/2
Neutron cross section (Brans): 145
Neutron Mass Absorption: 0.063
Isotopes: 99Rh 101mRh 101Rh 102mRh 102Rh 103Rh 105Rh
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 99Rh | Syn | – | 16.1 d |
| 101mRh | Syn | – | 4.34 d |
| 101Rh | Syn | – | 3.3 y |
| 102mRh | Syn | – | 3.7 y |
| 102Rh | Syn | – | 207 d |
| 103Rh | 100 | 102.905 | Stable |
| 105Rh | Syn | – | 35.36 h |
Chemical Reactions
Rhodium does not react with air under normal conditions, But If heating with oxygen at 600°C:
4 Rh (s) + 3 O2 (g) → 2 Rh2O3 (s) [dark grey] (Rhodium (III) oxide)
The metal reacts with all Halogens:
reacts directly with fluorine gas to form the highly corrosive,
Rh (s) + 3 F2 (g) → RhF6 (s) [black] (rhodium(VI) fluoride, Highly corrosive)
If heated, the hexafluoride is transformed to:
4 RhF6 (s) → [RhF5]4 (s) [dark red] + 2 F2 (g) (tetrameric rhodium(V) fluoride)
Under dry condition:
2 Rh (s) + 3 F2 (g) → 2 RhF3 (s) [red]
2 Rh (s) + 3 Cl2 (g) → 2 RhCl3 (s) [red] (Rhodium (lll) chloride)
2 Rh (s) + 3 Br2 (g) → 2 RhBr3 (s) [red-brown] (Rhodium (lll) bromide)
The reduction of nitrogen oxides to nitrogen and oxygen
2 NOx → x O2 + N2
Rhodium History
Naming: Greek: rhodon (rose). Its salts give a rosy solution.
Discovery and First isolation: William Hyde Wollaston (1804) in London
Rhodium Uses
Primary use of Rhodium is as an alloying agent to harden palladium and platinum.
These alloys are used in furnace windings, pen nibs, thermo coupling elements, headlight reflectors, phonograph needles, bearing, bushings for glass fiber production, electrodes for aircraft spark plugs, and laboratory crucibles.
It is useful as an electrical contact material as it has a low electrical resistance, a low and stable contact resistance, and is highly corrosion resistant.
Rhodium is used in catalytic converters for cars, where it reduces nitrogen oxides (NOX) in exhaust gases.
It is also used for jewelry, for decoration, and as a catalysts in chemical industry for making Nitric acid (HNO3), Acetic acid (CH3COOH) and hydrogenation reactions.
Plated rhodium is produced by electroplating or evaporation, which is exceptionally hard and is used for optical instruments (to coat optical fibers and optical mirrors).

Biological role: It is a suspected carcinogen. Exposure (contact) to rhodium (metal fume and dust, as Rh) should not exceed 1 mg/m3(8-hour time-weighted average, 40-hour week).
Abundance of Rhodium
Rhodium occurs uncombined in nature, along with other platinum metals in river sands of North and South America.
It is also found with other platinum metals in the copper-nickel sulfide, area of the Sudbury, Ontario region (Canada).
It is obtained commercially as a by-product of copper and nickel refining.
Annual world wide production is around 30 tons.
0.6×10-7% (In Universe)
1.8×10-5% (In Meteorites)
2×10-7% (In Sun)
7×10-8% (In Earth’s Crust)
World’s Top 3 producers of Rhodium
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Rhodium
1) South Africa
2) Russia
3) USA
#Rhodium


