74 W (Tungsten)

Impure metal is brittle and worked only with difficulty, and Pure tungsten is a lustrous, steel-gray to tin-white metal, which can be Spun, drawn, forged, extruded, and cut with a hacksaw.
It is the heaviest, and most refractory (resistant to heat) metal on the earth.
It has the highest tensile strength at the temperature over 1650 oC.
The metal oxidizes (combine chemically with oxygen.) in the air, and it must be protected at elevated temperatures.
Because of excellent corrosion resistance property, most mineral acids are very slightly attacks on the metal.
The thermal expansion is about the same as borosilicate (with use of silica & boron trioxide) glass, which makes the metal useful for glass-to-metal seals.
Identity
CAS Number: CAS7440-33-7
CID Number: CID23964
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSYO7175000
CONTENT INDEX
Basic Properties of Tungsten
Alternative name: Wolfram, pronounced
Pronunciation: Tung-stan
Appearance: Grayish white, lustrous
Mass Number: 184
Standard Atomic weight: 183.84 g/mol
Atomic number (Z): 74
Electrons: 74
Protons: 74
Neutrons: 110
Period: 6
Group: 6
Block: d
Element category: Transition metal
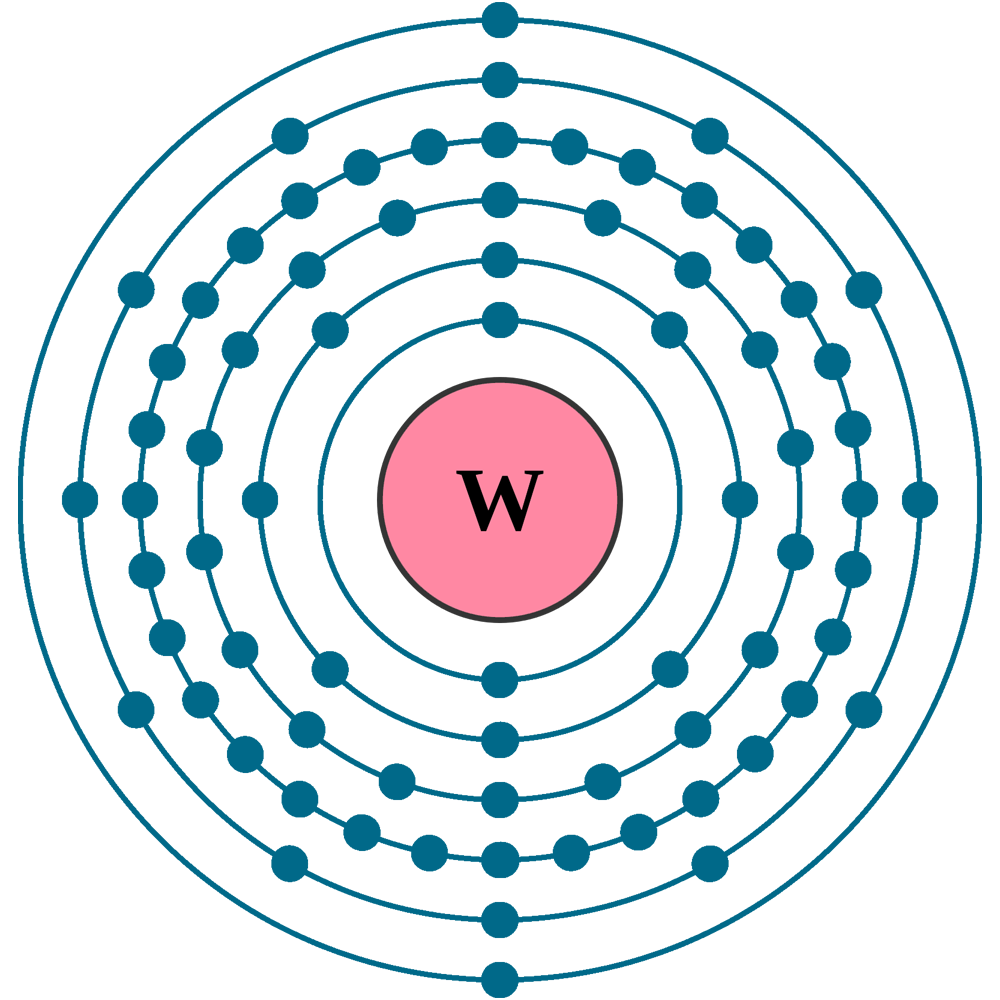
Electrons per shell: K2, L8, M18, N32, O12, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d46s2
Thermal Properties of Tungsten
Phase: Solid
Melting point: 3695 K (3422 oC, 6192 oF)
Boiling point: 6203 K (5930 oC, 10706 oF)
Debye temperature: 310 K (36.85 oC, 98.33 oF)
Fusion heat: 52.30 kJ/mol
Vaporization heat: 775 kJ/mol
Specific heat: 132 J/(kg K)
Molar heat capacity: 24.27 J/(mol.K)
Thermal expansion: 4.5 μm/(m∙K)
Thermal conductivity: 173 W/(m∙K)
Electrical properties of Tungsten
Electrical conductivity: 20×106 S/m
A Electrical resistivity: 52.8 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.015 K (-273.13 oC, -459.63 oF)
Magnetic Properties of Tungsten
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +59×10-6 cm3/mol
Volume magnetic susceptibility: 0.0000884
Mass magnetic susceptibility: 4.59×10-9 m3/kg
Molar magnetic susceptibility: 0.844×10-9 m3/mol
Physical Properties of Tungsten
Density: 19.3 g/cm3 (In solid) 17.6 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000095501 m3/mol
Young’s modulus: 411 GPa
Shear modulus: 161 GPa
Mohs Hardness: 7.5
Bulk modulus: 310 GPa
Poisson ratio: 0.28
Vicker hardness: 3430-4600 MPa
Brinell hardness: 2000-4000 MPa
Sound Speed: 4620 m/s
Atomic Properties of Tungsten
Oxidation states: -4, -2, -1, 0, 1, 2, 3, 4, 5, 6
Valence Electrons: 5d4 6s2
Ion charge: W6+
Ionization energies: 1st: 770 kJ.mol 2nd: 1700 kJ/mol
Ionic radius: 62 pm
Atomic radius: 139 pm (empirical)
Van der Waals: 210 Pm
Covalent radius: 162±7 pm
Filling Orbital: 5d4
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 316.52, 316.52, 316.52 pm
Grid parameters: a=3.160 Å
Space Group Name: lm_3m
Space Group Number: 229

Reactivity of Tungsten
Electronegativity: 2.36 (pauling scale)
Valence: +6
Electron affinity: 78.6 kJ/mol
Nuclear Properties of Tungsten
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 5D0
Neutron cross section (Brans): 18.4
Neutron Mass Absorption: 0.0036
Isotopes: 180W 181W 182W 183W 184W 185W 186W
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 180W | 0.12 | 179.946 | 1.8×1018 y |
| 181W | Syn | – | 121.2 d |
| 182W | 26.50 | 181.949 | Stable |
| 183W | 14.31 | 182.948 | Stable |
| 184W | 30.64 | 183.950 | Stable |
| 185W | Syn | – | 75 d |
| 186W | 28.43 | 185.952 | Stable |
Chemical Reactions of Tungsten
Finely divided tungsten metal is pyrophoric (liable to ignite spontaneously).
The metal doesn’t react with air under normal temperature, but at elevated temperature (red hot), Tungsten trioxide will form:
2W (s) + 3 O2 (g) → 2 WO3 (s)
Doesn’t react with water at room temperature:
Reacts directly with fluorine at room temp., and forms tungsten (VI) fluoride):
W (s) + 3 F2 (g) → WF6 (g) [colourless]
Reacts with other Halogens requies heat, at least 250 oC, and it forms tungsten (VI) halides:
W (s) + 3 Cl2 (g) → WCl6 (s) [dark blue] (tungsten (VI) chloride)
2 W (s) + 5 Cl2 (g) → 2 WCl5 (s) [dark green] (tungsten (V) chloride)
W (s) + 3 Br2 (g) → WBr6 (s) [dark blue] (tungsten (VI) Bromide)
W (s) + 3 I2 (g) → WI6 (s) (tungsten (VI) iodide)
The metal doesn’t react with most acids, under normal conditions.
Tungsten History
Naming: Swedish: tung sten (heavy stone): W symbol from its German name wolfram which is named after wolframite.
Naming by: Torbern Bergman (1781)
Discovery: Carl Wilhelm Scheele (1781) in vergara, spain
First isolation: Juan Jose Elhuyar & Fausto Elhuyar (1783)
Tungsten Uses
Tungsten its alloys are used extensively as the filament of electric light bulbs, electron and television tubes, and for metal evaporation work.
But these uses have been phased out from many countries, because of they are not very energy efficient, even they produce much more heat than light.
Tungsten has the highest melting point (3400 oC) from all other metals and it is alloyed with other metals to give strengthen them.
It is used in many high temperature applications, such as Tungsten arc welding (TIG) electrodes, heating elements in high-temperature furnaces etc..
Tungsten carbide (WC) is immensely (extremely) hard and it is made by mixing of tungsten & carbon powder, and heating to 2200°C.
It is very important to the metal-working, where It makes excellent cutting and drilling tools, including a new ‘painless’ dental drill which spins at ultra-high speeds.
It also mainly used in petroleum industries, and mining.
Magnesium tungstates (Magnesium dioxide tungsten, MgWO4) and Calcium are widely used in fluorescent lighting.
It is also used in X-ray tubes, which have tungsten emitter coil, where the screen used to view X-rays rely (depend on with full trust) on calcium & magnesium tungstates phosphors to convert X-rays into the blue visible light.
Other tungsten salts are used in the chemical and tanning industries, where the Tungsten disulfide (WS2) is a dry and high-temperature lubricant, which is stable to 500 oC.
Tungsten bronzes & other tungsten compounds are used in paints.
It is also used in microchip tecnology and liquid crystals displays (LCD).
Biological role of Tungsten
It is non-toxic metal, but its compound may have primary health risks, as like illitation to the skin & eyes on contact, inhalation of dust can cause irritation to the lungs and mucus membrane.
Some bacteria use tungsten in an enzyme to reduce carboxylic acids (an organic acid containing a carboxyl group) to aldehydes (organic compound containing a functional group with –CHO structure).
Abundance of Tungsten
The principal tungsten containing ores are wolframite ((Fe,Mn) WO4), scheelite (CaWO4), and Ferberite (FeWO4).
Commercially, The metal is obtained by reducing tungsten oxide (WO2) with carbon or hydrogen.
Annual world wide production is around 90,000 tons.
5×10-8% (In Universe)
1.2×10-5% (In Meteorites)
4×10-7% (In Sun)
0.00011% (In Earth’s Crust)
1.2×10-8% (In Oceans)


World’s Top 3 producers of Tungsten
1) China
2) Russia
3) Bolovia
World’s Top 3 Reserve holders of Tungsten
1) China
2) Russia
3) USA
Tungsten Price: Pure (99.95%) metal price is around $149 per KG (KiloGram)
#Tungsten


