117 Ts (Tennessine Element)

CONTENT INDEX
About Tennessine Element
Tennessine is a highly radioactive metal, of which only a few atoms have ever been made. It has Unknown chemical properties, presently it is placed in Halogen but probably a post-transition metal. The name Tennessine is used as a placeholder, This is a Latinate way of saying “one-one-seven-ium” (“ium” is a standard end for element names). Such transuranic elements are always produced artificially and are usually named for a scientist.
Identity
CAS Number: CAS587658-56-8
CID Number: N/A
DOT Hazard Class: N/A
DOT Number: N/A
RTECS Number: N/A
Properties of Tennessine Element
Basic Properties of Tennessine
Pronunciation: Ten-a-seen
Appearance: Semimetallic (predicted)
Mass Number: 294 (most stable Isotope)
Standard Atomic weight: 293 g/mol
Atomic number (Z): 117
Electrons: 117
Protons: 117
Neutrons: 177
Period: 7
Group: 17
Block: P
Element category: Unkown chemical properties (probably a Post-transition metal), But Presently consider in Halogen
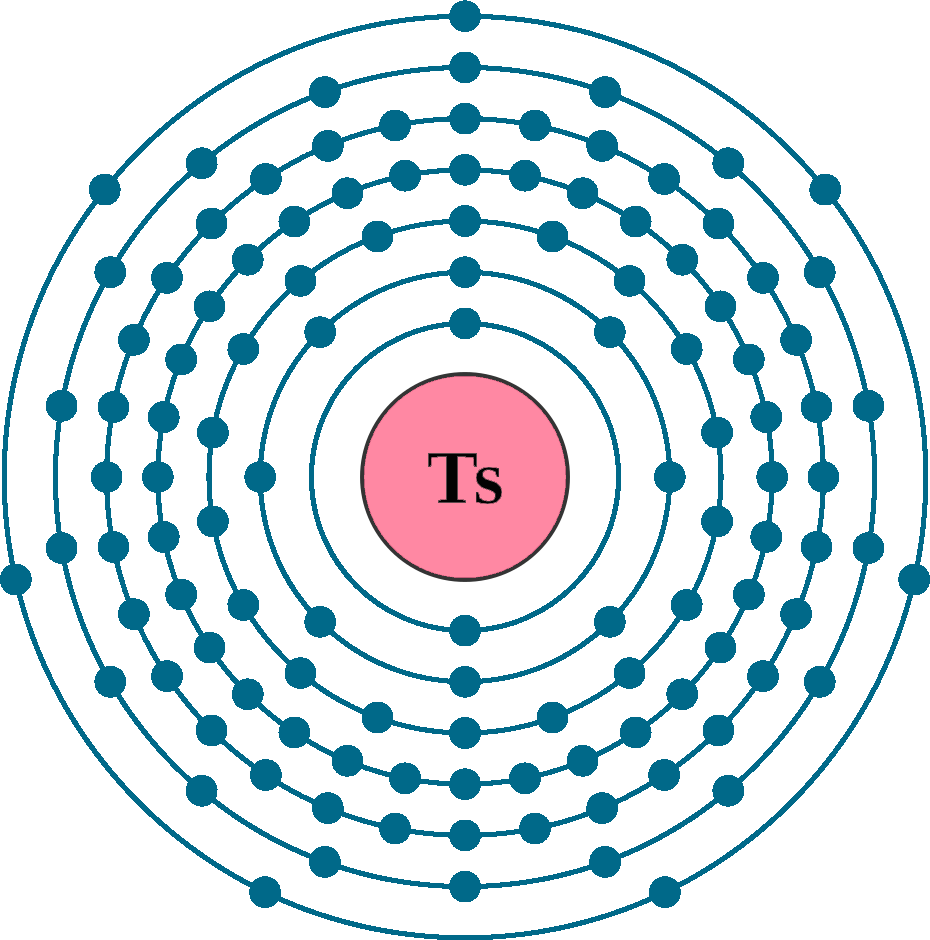
Electrons per shell: K2, L8, M18, N32, O32, P18, Q7
Electron configuration: 1s12s22p63s23p63d104s24p64d105s25p64f145d106s26p65f146d107s27p5
Thermal Properties of Tennessine Element
Phase: Solid (predicted)
Melting point: 623-823 K (350-550 oC, 662-1022 oF) (predicted)
Boiling point: 883 K (610 oC, 1130 oF)
Debye temperature: N/A
Triple Point Temperature: N/A
Triple Point Pressure: N/A
Critical point Temperature: N/A
Critical Point Pressure: N/A
Fusion heat: N/A
Vaporization heat: N/A
Specific heat: N/A
Molar heat capacity: N/A
Thermal expansion: N/A
Thermal conductivity: N/A
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Tennessine
Electrical conductivity: N/A
Electrical resistivity: N/A
Electrical type: N/A
Critical point (Superconducting point): N/A
Magnetic Properties of Tennessine
Magnetic type: N/A
Curie point: N/A
Magnetic susceptibility (xmol): N/A
Volume magnetic susceptibility: N/A
Mass magnetic susceptibility: N/A
Molar magnetic susceptibility: N/A
Physical Properties of Tennessine
Density: 7.1-7.3 g/cm3 (extrapolated)
Molar volume: N/A
Young’s modulus: N/A
Shear modulus: N/A
Mohs Hardness: N/A
Bulk modulus: N/A
Poisson ratio: N/A
Vicker hardness: N/A
Brinell hardness: N/A
Refractive Index: N/A
Sound Speed: N/A
Atomic Properties of Tennessine
Oxidation states: -1, +1, +3, +5 (predicted)
Valence Electrons: N/A
Ion charge: N/A
The ionization potential of an atom: N/A
Ionization energies: 1st: 742.9 kJ/mol (predicted) 2nd: 1435.5 kJ/mol (predicted) 3rd: 2161.9 kJ/mol (Predicted)
Ionic radius: N/A
Atomic radius: 138 pm (empirical) (predicted)
Van der Waals: N/A
Covalent radius: 157 pm (predicted)
Filling Orbital: N/A
Crystal structure: N/A
Lattice angles: N/A
Lattice constant: N/A
Grid parameters: N/A
Attitude c/a: N/A
Space Group Name: N/A
Space Group Number: N/A
Reactivity of Tennessine
Electronegativity: N/A
Valence: N/A
Electron affinity: N/A
Nuclear Properties of Tennessine Element
Half Life: 80 ms
Lifetime: 120 ms
Decay Mode: N/A
Quantum Number: 2P3/2
Neutron cross section (Brans): N/A
Neutron Mass Absorption: N/A
Isotopes: 293Ts 294Ts
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 293Ts | Syn | – | 22 ms |
| 294Ts | Syn | 294.210 | 51 ms |
Chemical Reactions of Tennessine Element
At Presently research is going on.
Tennessine History
Naming: After Tennessee region
Discover By: Scientists from Joint institute for Nuclear Research (JINR), in Dubna (Russia), Vanderbilt University, Lawrence Livemore National Laboratory (LLNL), In California (USA), and Oak Ridge National Laboratory (ORNL) (2010).
Discovery of Tennessine Element
In 2004, JINR proposed a joint experiment with ORNL to synthesize element 117. In this proposal they want fusion a berkelium-249 and a calcium beam via bombardment of the berkelium with calcium nuclei. But this experiment was suspended because obtaining the required amount of berkelium-249 was more difficult task than obtaining the californium-252, even it was very costly (around $3.5 Million).
In 2008, ORNL resumed production of californium and extraction of Berkelium, Because ORNL was the only producer of Berkelium. In 250 days they produced 22 miligrams of berkelium, which was enough to perform the experiment.
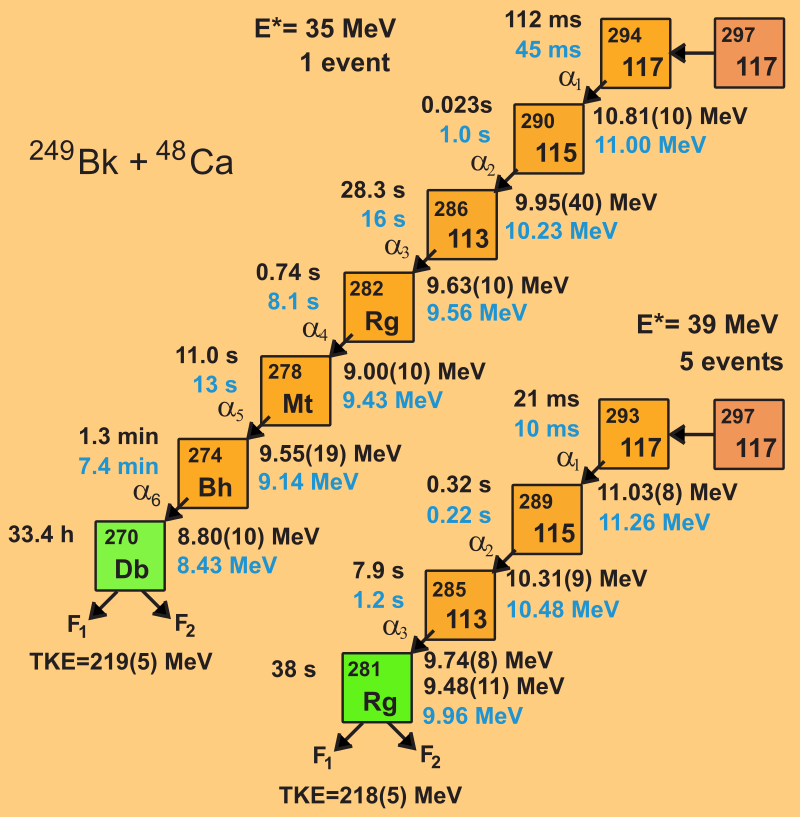
In 2009, in Research Institute of Atomic reactors (RIAR), berkelium deposited as a 300-nanometer thin layer on a titanium film and tranfered to JINR to installed in the Particle accelerator. Calcium beam was generated by extracting the small quantity of calcium-48, near at Lesnoy (Russia). In july 2009, scientists began the experiment and they had detected the decay of new element-117, via two decay chain. One was odd-odd isotope undergoing 6 α-decays before spontaneous fission, and one was odd-even isotope undergoing 3 α-decays before fission. The isotopes were formed as follows:
24997Bk + 4820Ca → 297117 + 3 10n (1 event)
24997Bk + 4820Ca → 297117 + 4 10n (5 event)
This data analysis provided by the support of JINR, LLNL, RIAR, ORNL, Vanderbilt, The University of Tennessee, and the University of Nevada.
In June 2016, International Union of Pure and Applied Chemistry IUPAC announced the name of discovered element 117 is Tennessine on the region of Tennessee.
Tennessine Uses
Since only a few atoms of Tennessine have ever been made, so it has no practical use outside of the scientific study, it is only used in research.
Biological role
It has no known biological role.
Sources of Tennessine Element
Unknown
Tennessine Element Database
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
→ Energy Levels of Hydrogen and Deuterium
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
→ Electrons (ESTAR)
→ Helium Ions (ASTAR)
→ Protons (PSTAR)
Nuclear Physics Data
→ Radionuclide Half-Life Measurements Data
→ Isotopic Compositions
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
National Institute of Standards and Technology
#tennessine


