01 H (Hydrogen Element)

CONTENT INDEX
About Hydrogen Element
Hydrogen is the first element of the periodic table. In normal condition, it is Odourless, Colourless and Tasteless gas, which is formed by diatomic molecules (H2). The hydrogen atom is formed by a nucleus with one unit of Proton (positive charge) and one electron (negative charge). It is one of the main compounds of water (H2O) and of all organic matter such as living plants, petroleum, coal etc…

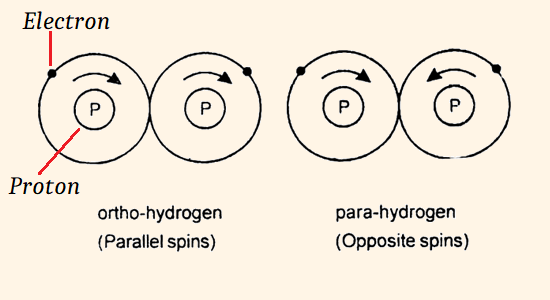
Apart from hydrogen isotopes, the ordinary hydrogen (H2) is the mixture of two kind of molecules, known as Ortho-hydrogen and Para-hydrogen. Which differ from another by nature of spin of their electrons and nuclei.
In Ortho-hydrogen, both the nuclei are spins in the same direction.
In Para-hydrogen, both the nuclei are spins in the opposite direction.
At the room temperature, hydrogen (H2) contains about 25% of the Para form and 75% of the ortho form.
There are Three isotopes of hydrogen, Hydrogen is the only element whose isotopes have been given different names.
1) Protium (1H) having 1 proton & 0 Neutron, it is the Ordinary isotope of Hydrogen (H), which is found in more than 99.984% of the natural element.
2) Deuterium (2H) having 1 Proton & 1 Neutron, which is found in nature about 0.015%, and 1 atom of Deuterium is found in about 6000 ordinary hydrogen atoms.
3) Tritium (3H) having 1 Proton & 2 Neutron, which appears in small quantities in nature, But it can be artificially produced by various nuclear reactions. It is used in the production of the Hydrogen bomb (fusion bomb). It is also used as a radioactive agent in making luminous paints, and as a Radioactive Tracer to diagnose industrial reactors.
Deuterium and Tritium are both used as fuel in nuclear fusion reactors.
Identity
CAS Number: CAS1333-74-0
CID Number: CID783
DOT Hazard Class: 2.1
DOT Number: 1966
RTECS Number: RTECSMW8900000
Properties of Hydrogen Element
Basic Properties of Hydrogen
Pronunciation: Hai-druh-jn / hai-dra-zen
Appearance: Colorless gas
Mass Number: 1
Standard Atomic weight: 1.008 g/mol
Atomic number (Z): 1
Electrons: 1
Protons: 1
Neutrons: 0
Period: 1
Group: 1
Block: S
Element category: Reactive Nonmetal
Electrons per shell: K1
Electron configuration: 1s1
Thermal Properties of Hydrogen
Phase: Gas
Melting point: 13.99 K (-259.16 oC, -434.49 oF)
Boiling point: 20.271 K (-252.879 oC, -423.182 oF)
Debye temperature: 110 K (-163.15 oC, -261.67 oF)
Triple Point Temperature: 13.803 K (-259.35 oC, -434.8 oF)
Triple Point Pressure: 7.041 kPa (0.0694 Atm)
Critical point Temperature: 32.938 K (-240.21 oC, -400.4 oF)
Critical Point Pressure: 1.2858 MPa (12.681 Atm)
Fusion heat: 0.117 kJ/mol (H2)
Vaporization heat (H2): 0.904 kJ/mol
Specific heat (H2): 14300 J/kg K
Molar heat capacity (H2): 28.84 J/(mol.K)
Thermal expansion: N/A
Thermal conductivity: 0.1805 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Hydrogen
Electrical conductivity: N/A
Electrical resistivity: N/A
Electrical type: N/A
Critical point (Superconducting point): N/A
Magnetic Properties of Hydrogen
Magnetic type: Diamagnetic
Curie point: N/A
Magnetic susceptibility (xmol): -3.98×10-6 cm3/mol
Volume magnetic susceptibility: -0.00000000223
Mass magnetic susceptibility: -0.248×10-9 m3/kg
Molar magnetic susceptibility: -0.004999×10-9 m3/mol
Physical Properties of Hydrogen
Density: 0.08988 g/L (at STP) 0.0763 g/cm3 (In solid) 0.07 g/cm3 (In Liquid at M.P) 0.07099 g/cm3 (In Liquid at B.P)
Molar volume: 0.01121 m3/mol
Young’s modulus: N/A
Shear modulus: N/A
Mohs Hardness: N/A
Bulk modulus: N/A
Poisson ratio: N/A
Vicker hardness: N/A
Brinell hardness: N/A
Refractive Index: 1.000132
Sound Speed: 1310 m/s
Atomic Properties of Hydrogen
Oxidation states: -1, +1
Valence Electrons: 1s1
Ion charge: H+ H–
The ionization potential of an atom: 13.53 eV
Ionization energies: 1st: 1312 kJ/mol
Ionic radius: 1.2 pm
Atomic radius: 53 pm (empirical)
Van der Waals: 120 Pm
Covalent radius: 31±5 pm
Filling Orbital: 1s1
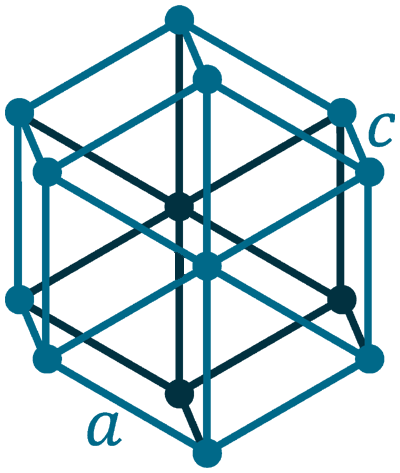
Crystal structure: Hexagonal
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 378.1, 378.1, 616.7 pm
Grid parameters: a(H)=3.780 Å c(H)=6.167 Å
Attitude c/a: 1.631
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Hydrogen
Electronegativity: 2.20 (pauling scale)
Valence: +1
Electron affinity: 72.5 kJ/mol
Nuclear Properties of Hydrogen
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 0.332
Neutron Mass Absorption: 0.011
Isotopes: 1H 2H 3H
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 1H | 99.98 | 1.008 | Stable |
| 2H | 0.02 | 2.015 | Stable |
| 3H | Trace | 3.014 | 12.32 y |
Chemical Reactions of Hydrogen
Reaction with Air
The element reacts with oxygen and with a flame or spark. The reaction of fire or explosion occurs, depending on the conditions.
2 H2 (g) + O2 (g) → 2 H2O (g)
Nitrogen can be react with hydrogen, which is called Haber process. Where heated with 400-5500 oC Fe catalyst.
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g)
Reaction with Water
Hydrogen doesn’t react with water, but slightly soluble in water.
Reaction with Halogens
Hydrogen reacts with halogens and forming hydrogen halides. The reactions are slow at normal temperature, except of fluorine and for increases the speed of reaction, increasing the temperature. Reaction of hydrogen with fluorine and chlorine can be explosive.
H2 (g) + F2 (g) → 2 HF (g)
H2 (g) + Cl2 (g) → 2 HCl (g)
H2 (g) + Br2 (g) → 2 HBr (g)
H2 (g) + I2 (g) → 2 HI (g)
Reaction with Acids & Bases
Hydrogen doesn’t react with dilute Acids & Bases
Reaction with Sulfur
The element reacts with sulfur and forms hydrogen sulfide.
S8 (s) + 8 H2 (g) ⇌ 8 H2S (g)
Reaction with Metals / Metal ions
Hydrogen reacts with alkali metals and strontium, calcium and barium, and forming Hydrides.
2 Li (s) + H2 (g) → 2 LiH (s)
2 Na (s) + H2 (g) → 2 Nah (s)
2 K (s) + H2 (g) → 2 KH (s)
2 Rb (s) + H2 (g) → 2 RbH (s)
2 Cs (s) + H2 (g) → 2 CsH (s)
Ca (s) + H2 (g) → CaH2 (s)
Sr (s) + H2 (g) → SrH2 (s)
Ba (s) + H2 (g) → BaH2 (s)
Cd is usually doesn’t not reacts with H2 at normal temperature. But At 450 ⁰C H2 I absorbed in the metal.
Cd (s) + H2 (g) → CdH (s) + H (reaction at 450 ⁰C)
Hydrogen does not react with Platinum, Gold, and Tungsten, but dissolved in the metal.
It reduces some metal oxides.
Fe3O4 (s) + H2 (g) → 3 Fe (s) + 4 H2O (g)
MnO4 (s) H2 (g) → MnO (s) + H2O (g)
Sun Reaction
2 11H → 21H + 01e + Heat Energy
11H + 21H → 32He + Gamma Waves
2 32He → 42He + 2 11H + Gamma Waves
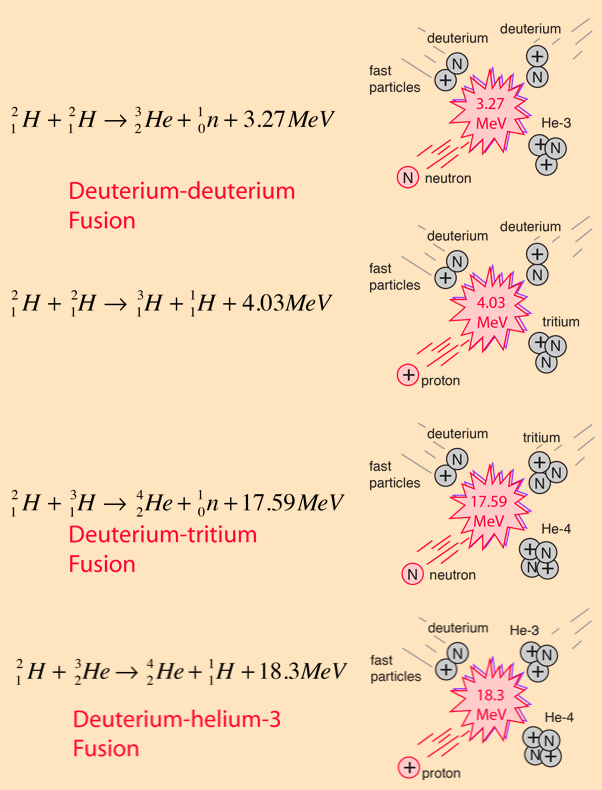
Fusion Reaction
(D=Deuterium, T=Tritium, B=Boron. Li=Lithium)
D + T → 4He + n + 17.58 MeV,
D + D → 3He + n + 3.27 MeV,
D + D → T + p + 4.03 MeV,
D + 3He → 4He + p + 18.35 MeV,
p + 11B → 3 4He + 8.7 MeV,
p + 7Li → 2 4He + 8.7 MeV
Tritium Generating from Lithium in Breeding reactors:
7Li + n → 4He + T + n –2.47 MeV,
6Li + n → 4He + T + 4.78 MeV.
History of Hydrogen Element
Naming: Greek: hydro (water) and genes (generate), Latine name = Hydrogenium
Discovery: Herry Cavendish (1766)
Naming by: Antoine Lavoisier (1783)
Hydrogen Uses
Large quantities of hydrogen are used in the Haber process for production of Ammonia for agricultural fertiliser, where the hydrogen reacted with nitrogen to form anhydrous liquid ammonia. Hydrogen is also largly used in Hydrogenate oils to form fats (for example to make margarine), in production of Methanol (CH3OH) and Cyclohexane (C6H12), in Hydrocracking, in Hydrodesulfurization (sulphur elimination), and in Hydrofining, in metal refining. Other uses are in the production of hydrochloric acid (HCL), in Welding, in filling ballons, and in the reduction of metallic ores.
Liquid hydrogen is used in cryogenic Research, including Superconductivity studies, as well as a Rocket fuel in Cryogenic engine to powering the Space Shuttle’s lift-off and ascent into orbit, and as a rocket propellent propelled by Nuclear energy.
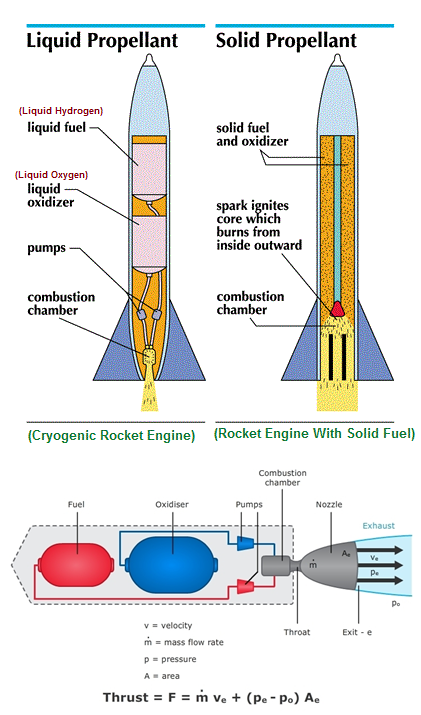
Credit this Image to university of waikato
Isotopes Deuterium and Tritium are used in Nuclear Fusion.
Isotope Deuterium (hydrogen-2) is used in Nuclear Fission applications as a moderator to slow down Neutrons, and is also used in nuclear fusion reactions. Deuterium compounds have applications in chemistry and biology in studies of reaction isotope effects.
Isotope Tritium (hydrogen-3) produced in nuclear reactors, and it is used to construct Hydrogen Bombs. It is also used as an isotopic label in the biosciences and as a radiation source in luminous paints.
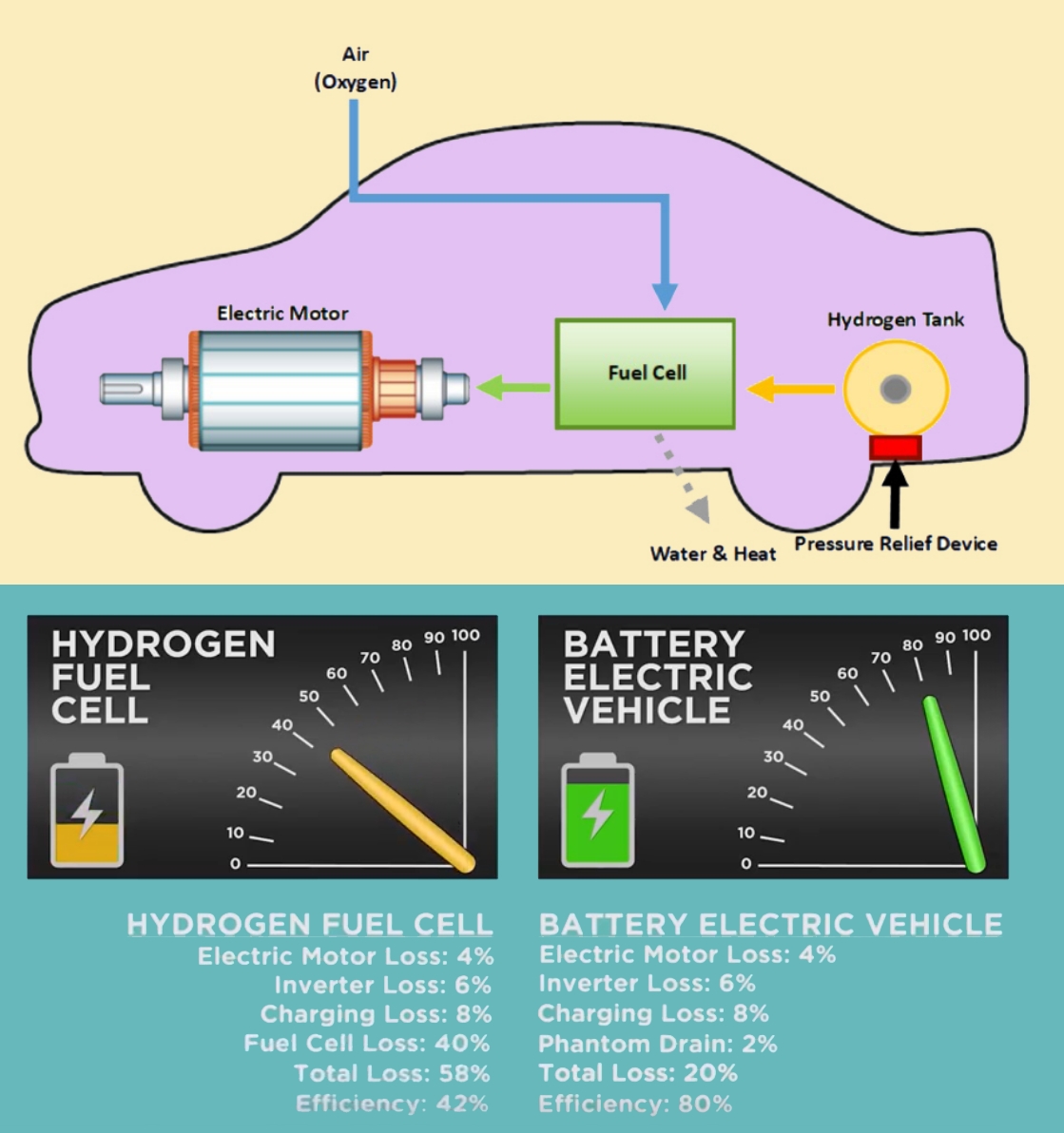
Hydrogen can be burned in internal combustion engines, and provide pollution-free power (no emissions of CO2 or toxic chemicals). This new technology of hydrogen is developing in form of “Hydrogen fuel cell”, that will allow great amounts of electrical power to be obtained using a source of hydrogen gas. Researchers see hydrogen gas as the cleanest fuel of the future because it generated from water and returning to water when it is oxidized. This Hydrogen-powered fuel cell technology are now being used in some buses and cars.
In the glass industry hydrogen is used as a protective atmosphere to make flat glass sheets, and In the electronics industry it is used as a flushing gas during the manufacture of silicon chips.
Hydrogen is about Fourteen times lighter than air, that’s why it was used for filling balloons and Airships, but it reacts vigorously with oxygen. So its future of filling airship ended after Hindenburg disaster in 1937, where Hindenburg airship caught fire.

Hydrogen compound H2O2 (hydrogen peroxide) is a mild antiseptic, it is used on wound or cut on skin to prevent infection, it can also be used to germ-free cleaning like in mouth wash, in Laundry additives, pit stain remover.
Biological role of Hydrogen Element
Hydrogen is an essential element for our life. It is present in water and in almost all the molecules in living things. However It remains bonded to carbon and oxygen atoms, while the chemistry of life occurs at more active sites, for example, nitrogen, oxygen, and phosphorus.
Hydrogen is extremely flammable and many reaction may cause fire or explosion. Heating the gas may cause violent combustion or explosion. It Reacts violently with air, oxygen, halogens and strong oxidants causing fire and explosion hazard. Metal catalysts, such as platinum and nickel, greatly enhance these reactions.
Hydrogen is non-toxic, but It could be very dangerous for you while inhalation, because of its explosive nature.
High concentrations of hydrogen cause an Oxygen-deficient environment, and breathing in such that environment may experience some symptoms that include headaches, ringing in the ears, drowsiness, nausea, fainting, dizziness, vomiting and depression of all senses. Even the skin may have a blue color and under some circumstances, death may occur.
First aid while Fire: Shut off the supply, if it is not possible and no risk to surroundings, then let the fire burn itself out. In other cases you can extinguish the fire with water spray, powder, or carbon dioxide (CO2).
First aid while Explosion: In case of fire, keep cylinder cool by spraying with water. Inhale Fresh air, where Artificial respiration may be needed, and Refer to medical attention.
Sources of Hydrogen Element
Hydrogen is the most abundant element in the universe, making up about 75% of the universe’s mass and over 90% of all atoms. Hydrogen is found in the largest amount in the water on the earth, despite its great abundance everywhere, hydrogen is very rare in the earth’s atmosphere (less than 1 part per million by volume). Because when hydrogen enters in the atmosphere of earth, then due to light weight quickly escapes from the Earth’s gravity into outer space.
The element is found in the Stars, which plays an important role in universe to given power through the both Carbon-Nitrogen-Oxygen cycle (CNO Cycle) and Proton-Proton reaction. Stellar hydrogen fusion processes (nuclear fusion of four protons to form a helium-4 nucleus) release enormous amounts of energy by combining hydrogens to form helium.
Melting point of hydrogen is very low, it’s only about 20 degrees above the absolute zero, so Liquid hydrogen is very important in cryogenics and in the study of Superconductivity.
Hydrogen Isotope (Tritium) is readily produced in nuclear reactors, which is used in the production of the Hydrogen Bomb.
Hydrogen is also found in great abundance in gas giant planets like Jupiter, where hydrogen is the primary component and at some depth of the planet, the pressure is so high, that converts the solid molecular hydrogen to solid metallic hydrogen.
Hydrogen can be Prepared By several ways:
1) Steam on heated carbon (steam-carbon reaction), where hydrogen atoms is separated from carbon atoms in methane (CH4).
CH4 + H2O + heat → CO + 3 H2
2) Decomposition of some hydrocarbons with heat.
3) Reaction of a strong base in an aqueous solution Like Caustic soda (sodium hydroxide, NaOH) with Aluminium.
Zn + 2 NaOH → Na2ZnO2 + H2
Pb + 2 NaOH → Na2PbO2 + H2
2 Al + 6 NaOH → 2 Na3AlO3 + 3 H2
4) Water Electrolysis, where splits the hydrogen from water (H2O) using an electric current.
2 H2O → 2 H2 + O2
5) Displace the hydrogen from acids with certain metals (Potassium, Sodium, Calcium, Magnesium, Aluminium, Zinc, Iron, Lead etc…), where Metals react with acids to produce salt and hydrogen.
Mg + 2 HCl → MgCl2 + H2 (Mg react in form of Mg2+ and HCl in form of H+Cl–)
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
Zn + 2HCl → ZnCl2 + H2 (Zinc reacts with dilute HCl to form zinc chloride and Hydrogen gas)
Zn + H2SO4 → ZnSO4 + H2 (Zinc reacts with dilute H2SO4 to form zinc sulphate and Hydrogen gas)
(Zinc is used to preparation Hydrogen in Laboratory Because zinc doesn’t react violently, and not very expensive)
6) Metals react with water to produced hydrogen
2 K + 2 H2O → 2 KOH + H2 (in this reaction K+ & H+OH– )
2Na + 2 H2O → 2 NaOH + H2
Ca + 2 H2O → Ca(OH)2 + H2
Sometimes some metals doesn’t react with cold water then
Mg + H2O (steam) → MgO + H2
Zn + H2O (steam) → Zno + H2
3 Fe + 4 H2) (steam) ⇌ Fe3O4 + 4 H2
Today, 95% of hydrogen is Commercial produced either from wood or by decomposing fossil fuels, such as natural gas and oil.
How to Get Pure Hydrogen from Impure hydrogen in Laboratory
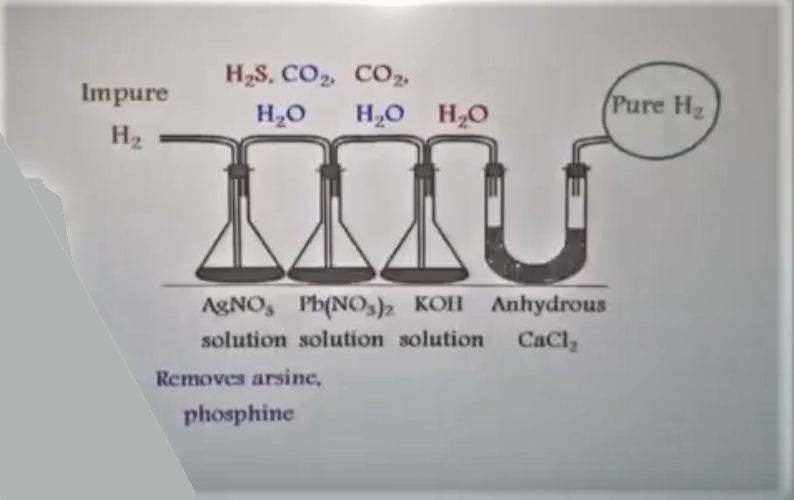
Industrial Hydrogen gas Preparation (Bosch Process)
C + H2O → (CO + H2) (water gas)
(CO + H2) + H2O → CO2 + 2 H2 (Impure Hydrogen)
2 KOH + CO2 → K2CO3 + H2O (Liquid and solid compound)
CuCl + CO + 2 H2O → CuCl.CO.2H2O
Research is underway to develop other methods of producing hydrogen such as
– Using microbes that use light to make hydrogen,
– Converting biomass into gas or liquids and separating the hydrogen,
– Using solar energy technologies to split hydrogen from water molecules.
Annual world wide production is around 300,000,000,000 cubic meters.
75% (In Universe)
2.4% (In Meteorites)
75% (In Sun)
0.15% (In Earth’s Crust)
11% (In Oceans)
10% (In Humans)
World’s Top 3 producers of Hydrogen
1) Unknown
2) Unknown
3) Unknown
World’s Top 3 Reserve holders of Hydrogen
1) Unknown
2) Unknown
3) Unknown
Hydrogen Element Price
Hydrogen price is around $2-$3 per Cubic meter.
Hydrogen Element Database
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
→ Energy Levels of Hydrogen and Deuterium
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
→ Electrons (ESTAR)
→ Helium Ions (ASTAR)
→ Protons (PSTAR)
Nuclear Physics Data
→ Radionuclide Half-Life Measurements Data
→ Isotopic Compositions
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements


