02 He (Helium Element)
CONTENT INDEX
About Helium Element
Helium is a Odourless, Colourless, insipid and non-toxic gas. It has many unique properties such as low density, low boiling point, low solubility, high thermal conductivity and inertness (less reactive).
It is the only element that cannot be solidified by lowering the temperature, It remains liquid down to absolute zero at normal pressures, but it can readily solidify by increasing the pressure.

The specific heat of helium is high and the density of helium gas is very low but at the normal boiling point is high. Soild Helium-3 (He3) and Helium-4 (He4) are unusual in that both can be changed in volume by more than 30% by applying pressure.
Helium is 6 times Lighter than the air, so when you inhale or breath some of the helium then the pitch of your voice goes up, and your voice will change just like Donald duck. Similarly Sulfur hexafluoride (SF6) also inert and non-toxic but it is 6 times heavier than the air, so when you inhale some of the SF6 then the pitch of your voice goes up, and your voice will change just like Darth Vader.
Valency of Helium is zero, so it seems to have a weak tendency to combine with certain other elements.

Identity
CAS Number: CAS7440-59-7
CID Number: CID23987
DOT Hazard Class: 2.2
DOT Number: 1963
RTECS Number: RTECSMH6520000
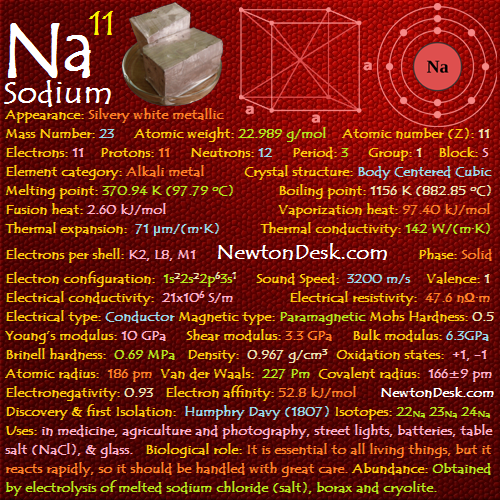
Properties of Helium
Basic Properties of Helium
Pronunciation: Hee-lee-am / hi-liam
Appearance: Colorless gas, exhibiting a gray, cloudy glow when placed in an electric field
Mass Number: 4
Standard Atomic weight: 4.002 g/mol
Atomic number (Z): 2
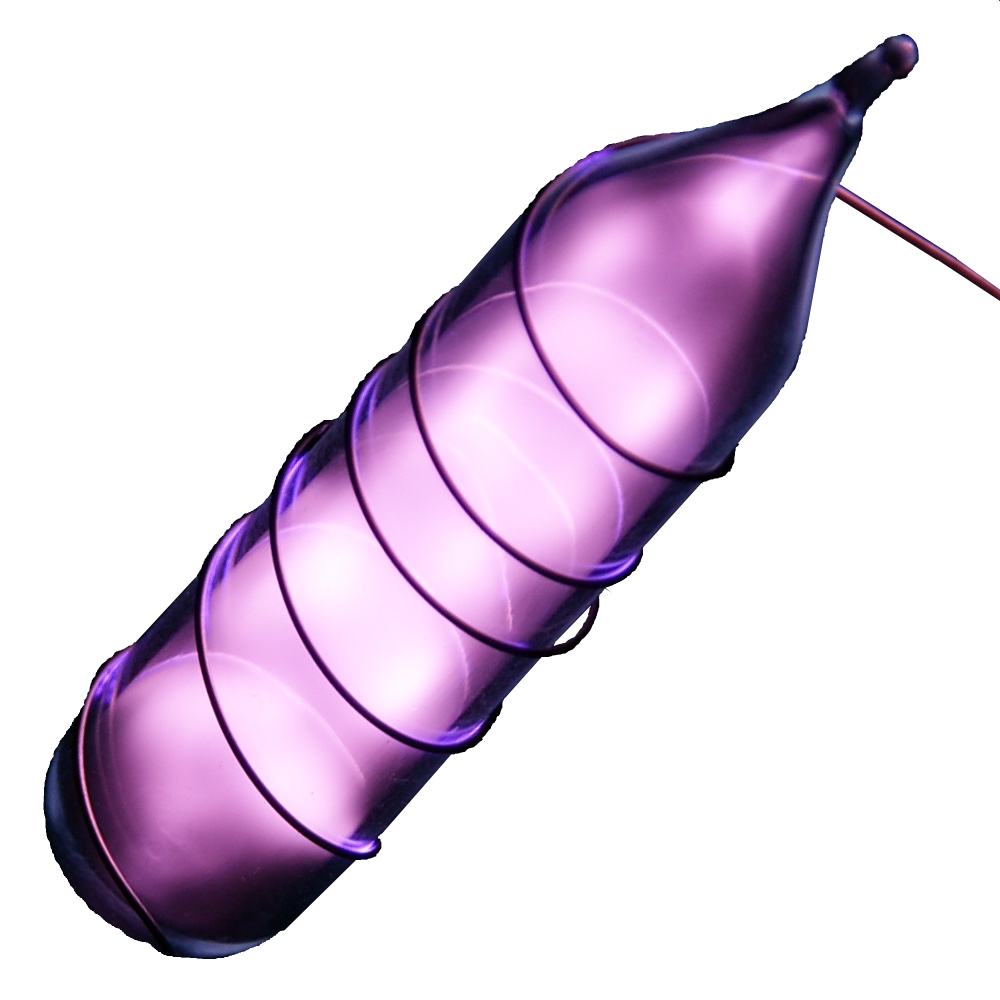
Electrons: 2
Protons: 2
Neutrons: 2
Period: 1
Group: 18
Block: S
Element category: Noble gas
Electrons per shell: K2
Electron configuration: 1s2
Thermal Properties of Helium
Phase: Solid
Melting point: 0.95 K (-272.20 oC, -457.95 oF)
Boiling point: 4.22 K (-268.92 oC, -452.07 oF)
Debye temperature: N/A
Triple point Temperature: 2.17 K (-270.98 oC, -455.76 oF)
Triple point Pressure: 5.043 KPa (0.0498 atm)
Critical point Temperature: 5.19 K (-267.96 oC, -450.32 oF)
Critical point Pressure: 0.22746 MPa (2.2448 atm.)
Fusion heat: 0.0138 kJ/mol
Vaporization heat: 0.0829 kJ/mol
Specific heat: 5193.1 J/(kg K)
Molar heat capacity: 20.78 J/(mol.K)
Thermal expansion: N/A
Thermal conductivity: 0.1513 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Helium
Electrical conductivity: N/A
Electrical resistivity: N/A
Electrical type: N/A
Critical point (Superconducting point): N/A
Magnetic Properties of Helium
Magnetic type: Diamagnetic
Curie point: N/A
Magnetic susceptibility (xmol): -1.88×10-6 cm3/mol
Volume magnetic susceptibility: -0.00000000105
Mass magnetic susceptibility: -5.9×10-9 m3/kg
Molar magnetic susceptibility: -236×10-9 m3/mol
Physical Properties of Helium
Density: 0.178 g/L (at STP) 0.145 g/cm3 (In Liquid at M.P)
Molar volume: 0.022424 m3/mol
Young’s modulus: N/A
Shear modulus: N/A
Mohs Hardness: N/A
Bulk modulus: N/A
Poisson ratio: N/A
Vicker hardness: N/A
Brinell hardness: N/A
Refractive Index: 1.000035
Sound Speed: 972 m/s
Atomic Properties of Helium
Oxidation states: 0
Valence Electrons: 1s2
Ion charge: N/A
The ionization potential of an atom: 24.47 eV
Ionization energies: 1st: 2372.2 kJ.mol 2nd: 5250.5 kJ/mol
Ionic radius: N/A
Atomic radius: 31 pm (empirical)
Van der Waals: 140 Pm
Covalent radius: 28 pm
Filling Orbital: 1s2
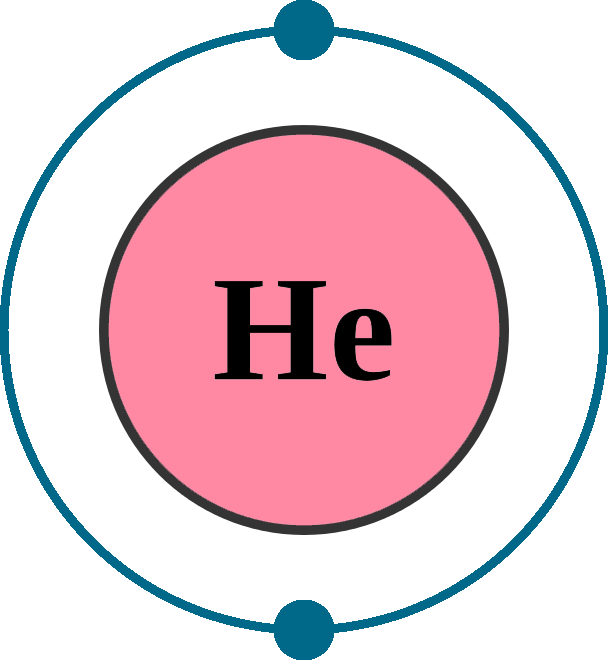
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 357, 357, 584 pm
Grid parameters: a(H)=3.570 Å c(H)=5.84 Å
Attitude c/a: 1.633
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Helium
Electronegativity: N/A
Valence: 0
Electron affinity: 0 kJ/mol
Nuclear Properties of Helium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Decay Mode: N/A
Quantum Number: 1S0
Neutron cross section (Brans): 0.007
Neutron Mass Absorption: 0.00001
Isotopes: 3He 4He
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 3He | 0.0002 | 0.000135 | Stable |
| 4He | 99.9998 | 99.9999 | Stable |
Chemical Reactions of Helium
It doesn’t react with Air, Water, Halogens, Acid, Bases etc…
Fission and Fusion Equations:
Fission Reaction of Uranium 235 (U-235) Bombarded by Neutrons:
10n + 23592U → 14156Ba + 9236Kr + 3 10n
Fusion Reaction of Two Helium-3 Atoms:
32He + 32He → 42He + 2 11P + 12.85 MeV or
22He + 32He → 42He + 11P + 18.3 MeV
Fusion of Deuterium and Helium-3:
D (21H) + 32He → 42He + 11P + 18.4 MeV
Helium History
Naming: After Helios, (Greek Titan of the sun)
Discovery: Pierre Janssen, Norman Lockyer (1868)
First isolation: Willian Ramsay, Per Teodor cleve, Abraham Langlet (1895), London (England) / Uppsala (Sweden)
Helium Uses
Helium is non reactive element, so it is used as an inert gas shield in robotic Arc welding. It is also used as a protective gas (non-reactive atmosphere) for growing Silicon & Germanium crystals and producing Titanium & Zirconium. Silicon and Germanium crystals are used to make fiber optics and electronic semiconductor devices.
Helium is the only element which is capable to reaching temperatures lower than 15 K ( 258.15ºC, -434ºF). That’s why Helium is used in Cryogenic research, and also used as a cooling medium for the Large Hadron Collider (LHC), as well as a working fluid in Nuclear reactors cooled down by gas, and for the superconducting magnets in MRI (magnetic resonance imaging) scanners and NMR Spectrometers, and in the development of the superconductivity state, where the resistance to the electricity flux is almost zero. It is also used to keep cool the Satellite instrument, Liquid oxygen, and Liquid Hydrogen.
Helium is used as a gas for Supersonic wind tunnels.
Because of its low density, Helium is very light weighted gas and is often used to fill Decorative balloons, Weather balloons, and Airships (dirigibles ballons). Hydrogen is also very light but can’t used in these applications, because it is dangerously reactive, and helium is much safer than hydrogen.
Helium is also used as Mass Spectrometer Leak Detector (MSLD) to detect leaks, such as in car air-conditioning systems, and because of it diffuses (spread) quickly, it is used in the Car Airbags to inflate (fill with air or gas) after impact.
Helium is used as pressurizing gas in Liquid propellant (fuel) for Rockets, and in a Helium(80%)-Oxygen(20%) mixture, which is used as an artificial atmosphere for deep-sea divers and others working. Ratios of He and O2 can be changes for different diver opration depths.
Helium(He)-neon(Ne) gas lasers are used to scan barcodes in supermarket checkouts.
Helium is now also used in helium-ion microscopes that gives better image resolution than scanning electron microscopes.
Now our scientists are working on the isotope helium-3, because Helium-3 (He3) gas has the potential to be used as a fuel in future Nuclear fusion (Aneutronic fusion) power plants. But helium-3 is very rare on earth, even It is produced as a by-product of the maintenance of nuclear weapons. Scientist expect that a huge amount of Helium-3 is available on moon, because the Moon has been bombarded with large quantities of Helium-3 by the solar wind. Several space agencies have subsequently signalled their intention to go to the Moon to mine helium-3 as a fuel supply.

ISRO, Indian Space Research Organization’s mission Chandrayan-2 was also launched on 22 July 2019, which has to be land on 7 sept.2019 to discover the Helium-3 on the southern side of the moon, but unfortunately, it’s lander was crashed on the surface of moon after breaking the contact before 2.1 KM Altitude. But orbiter is going working fine, which having 8 Payloads to study of the moon.
Sun + Cosmic rays = Helium-3
Biological role of Helium
Helium has no biological role, and at standard conditions It is non-toxic.
Effects on normal exposure, The substance can be absorbed into the body by inhalation. But Inhalation on High amount, then Dizziness, Dullness, Headache, Suffocation can occur. It is only risked in that area, where lower oxygen is contained in the air, that’s why Check oxygen content before entering the area.
Abundance of Helium
After the hydrogen, helium is the 2nd most abundant element found in the universe. It is extracted from the Natural gas, which can contain up to 7% helium. In fact, all-natural gases contain at Least trace quantity of helium and the concentration of helium is higher than the atmosphere.
Helium is present in great abundance, in all hotter stars, and it is an important component in both the proton-proton reaction and the carbon cycle, which is responsible for the energy of the sun and stars.
Helium is still being formed underground from alpha (α) particle decay of heavy radioactive elements (such as Uranium and Thorium) in The Earth.
Some of the helium migrates to the surface and enters into the atmosphere, which contains about 5 parts per million by volume. Due to low-density helium quickly rise and escapes into outer space, and It is uneconomical to extract helium from the air.
Annual world wide production is around 180,000,000 (180 Million) Cubic Meters, and World wide Reserve is around 45,000,000,000 (45 Billion) Cubic Meters.
23% (In Universe)
N/A (In Meteorites)
23% (In Sun)
5.5×10-7% (In Earth’s Crust)
47.2×10-10% (In Oceans)
N/A (In Humans)
World’s Top 3 producers of Helium
1) USA
2) Algeria
3) Russia
World’s Top 3 Reserve holders of Helium
1) USA
2) Qatar
3) Algeria
Helium Price
Pure (99.995%) element price is around $10-$20 per Litre.
Element Database
Atomic Spectroscopic Data
→ ASD Line
→ Ground States and Ionization Energies
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
Nuclear Physics Data
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Los Alamos National Laboratory


Dear author
Did you know that JUPITER became almost a second sun.
But the gas was not condensed enough to cause a reaction.
Humans in the future may be able to create a 2. nd sun. In our solar system.
Even transporting an entire planet into another solar system is possible. But only the most extreme genuiuses could do that. Individuals who know 1000000% what they are doing.
Note there have been supernovas ; that means other lives on other Solarsystems lived billion of years ago.
Our planet is one of the last evolving planets