20 Ca (Calcium Element)

Calcium has a silvery color metal, which is harder than sodium, and softer than aluminium.
It is less chemically reactive than the alkaline and other alkaline-earth metals.
It readily forms a white coating of nitride in air, which protects it from further corrosion, and it burns in air with a yellow-red flame.
The metal reacts easily with water and acids and produces hydrogen, which contains remarkable amounts of hydrocarbides & ammonia as impurities.

Identity
CAS Number: CAS7440-70-2
CID Number: CID5460341
DOT Hazard Class: 4.2
DOT Number: 1855
CONTENT INDEX
Basic Properties of Calcium
Appearance: Silver, dull gray (with a pale yellow tint)
Mass Number: 40
Standard Atomic weight: 40.078 g/mol
Atomic number (Z): 20
Electrons: 20
Protons: 20
Neutrons: 20
Period: 4
Group: 2
Block: s
Element category: Alkaline earth metal
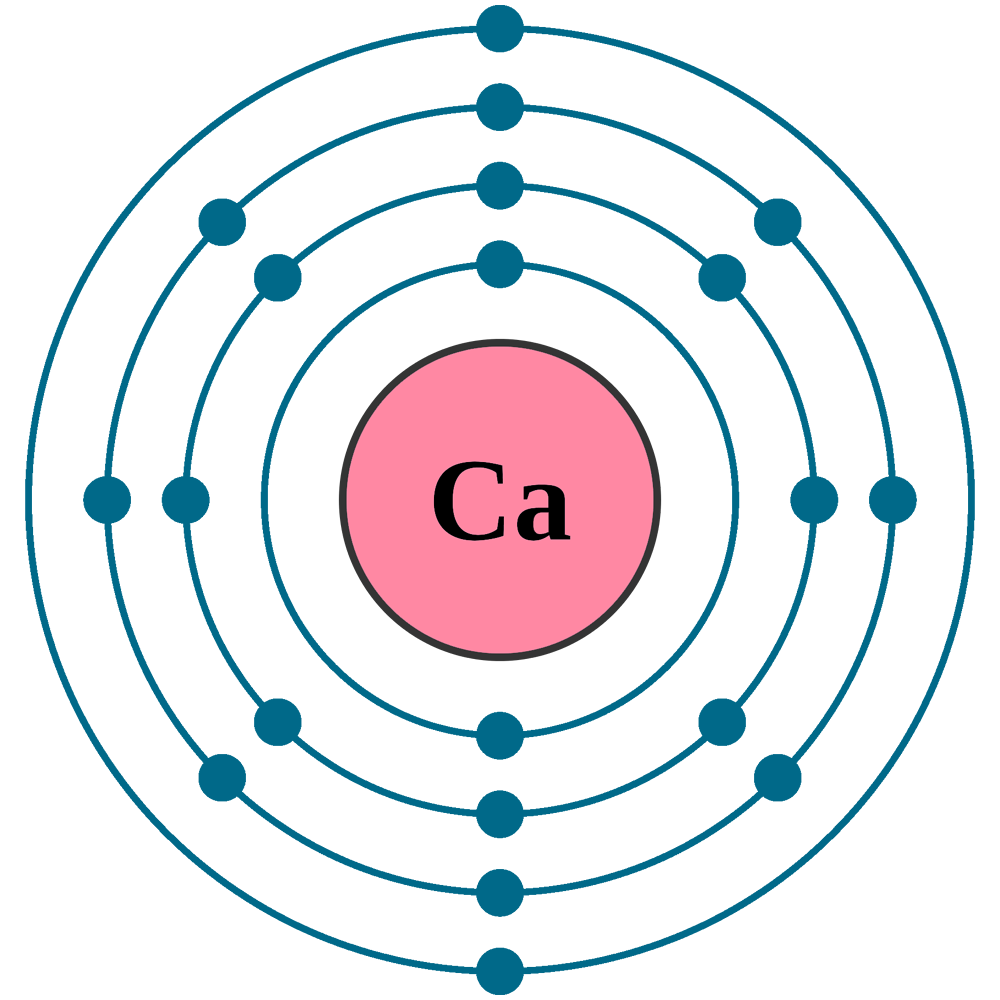
Electrons per shell: K2, L8, M8, N2
Electron configuration: 1s22s22p63s23p64s2
Thermal Properties of Calcium
Phase: Solid
Melting point: 1115 K (842 oC, 1548 oF)
Boiling point: 1757 K (1484 oC, 2703 oF)
Debye temperature: 230 K (-43.15 oC, -45.67 oF)
Fusion heat: 8.54 kJ/mol
Vaporization heat: 154.8 kJ/mol
Specific heat: 631 J/(kg K)
Molar heat capacity: 25.929 J/(mol.K)
Thermal expansion: 22.3 μm/(m∙K)
Thermal conductivity: 201 W/(m∙K)
Electrical properties of Calcium
Electrical conductivity: 29×106 S/m
A Electrical resistivity: 33.6 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Calcium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): +40×10-6 cm3/mol
Volume magnetic susceptibility: 0.00002139
Mass magnetic susceptibility: 13.8×10-9 m3/kg
Molar magnetic susceptibility: 0.553×10-9 m3/mol
Physical Properties of Calcium
Density: 1.55 g/cm3 (In solid) 1.378 g/cm3 (In Liquid at M.P)
Molar volume: 0.000025857 m3/mol
Young’s modulus: 20 GPa
Shear modulus: 7.4 GPa
Mohs Hardness: 1.75
Bulk modulus: 17 GPa
Poisson ratio: 0.31
Brinell hardness: 170-416 MPa
Sound Speed: 3810 m/s
Atomic Properties of Calcium
Oxidation states: +1, +2
Valence Electrons: 4s2
Ion charge: Ca2+
Ionization potential of an atom: 6.09
Ionization energies: 1st: 589.9 kJ.mol 2nd: 1145 kJ/mol 3rd: 4912.5 kJ/mol
Ionic radius: 99 pm
Atomic radius: 197 pm (empirical)
Van der Waals: 231 Pm
Covalent radius: 176±10 pm
Filling Orbital: 4s2
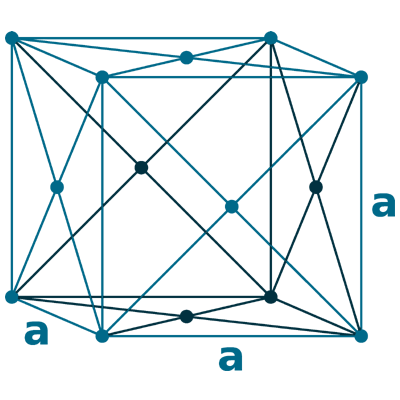
Crystal structure: Face centred cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 558.2, 558.2, 558.2 pm
Grid parameters: a=5.580 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Calcium
Electronegativity: 1.00 (pauling scale)
Valence: +2
Electron affinity: 2.37 kJ/mol
Nuclear Properties of Calcium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 7F0
Neutron cross section (Brans): 5900
Neutron Mass Absorption: 4.7
Isotopes: 40Ca 41Ca 42Ca 43Ca 44Ca 45Ca 46Ca 47Ca 48Ca
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 40Ca | 96.941 | 39.964 | Stable |
| 41Ca | Trace | – | 1.03×105 y |
| 42Ca | 0.647 | 41.958 | Stable |
| 43Ca | 0.135 | 42.959 | Stable |
| 44Ca | 2.086 | 43.956 | Stable |
| 45Ca | Syn | – | 162.7 d |
| 46Ce | 0.004 | 45.955 | Stable |
| 47Ce | Syn | – | 4.5 d |
| 48Ce | 0.187 | 47.953 | 6.4×1019 y |
Chemical Reactions of Calcium
The metal reacts with oxygen at room temp., and forming a thin layer of CaO, which protect the metal from further oxidation.
The metal once ignited, it burn in air, and forms a mixture of white calcium oxide (CaO) & calcium nitride (Ca3N2):
2 Ca (s) + O2 → 2 CaO (s)
3 Ca (s) + N2 (g) → Ca3N2 (s)
Reacts slowly with water, and forming calcium hydroxide & hydrogen:
Ca (s) + 2 H2O (l) → Ca(OH)2 (aq) + H2 (g)
Reacts with halogens, and forming Calcium (II) halides,where the reactions with fluorine & chlorine are exothermic, and with bromine & iodine are endothermic:
Ca (s) + F2 (g) → CaF2 (s) (Calcium (II) fluoride)
Ca (s) + Cl2 (g) → CaCl2 (s) (Calcium (II) chloride)
Ca (s) + Br2 (g) → CaBr2 (s) (Calcium (II) bromide)
Ca (s) + I2 (g) → CaI2 (s) (Calcium (II) iodide)
Ca dissolves readily in dilute or concentrated hydrochloric acid (HCl), and forms Ca (II) ions & hydrogen gas:
Ca (s) + 2 HCl (aq) → Ca2+ (aq) + 2 Cl– (aq) + H2 (g)
Production:
In reaction forms Ca2+ with bicarbonate ion (HCO3–), by CO2 with water at seawater, which contain calcium carbonate::
CaCO3 (s) + CO2 + H2O → Ca2+ + 2 HCO3–
Other Reactions:
Ca ions are precipitated by carbonate ions in netural to alkaline solution:
Ca2+ (aq) + CO32- (aq) → CaCO3 (s) [white]
ACa ions can be precipitated by strong bases:
Ca2+ (aq) + 2 OH– (aq) → Ca(OH)2 (s)
Precipitated by phosphate ions in alkaline solutions:
5 Ca2+ (aq) + 3 PO43- (aq) + OH– (aq) → Ca5(SO4)3OH (s) [white]
Ca ions are precipitated by sulfate ions in neutral to alkaline solutions.
Ca2+ (aq) + SO42- (aq) → CaSO4 (s) [white]
Calcium History
Naming: Latin: calx, calcis (lime).
Discovery & first isolation: Humphry Davy (1808) in London, England
Calcium Uses
ACalcium metal is used as a reducing agent (element or compound that donates electron to another chemical species) in preparing other metals such as uranium , thorium, zirconium etc.., and is used as a deoxidizer, desulfurizer, or decarburizer (compound used in a reaction to remove oxygen, sulfur,or carbon) for various ferrous and nonferrous alloys.
Calcium silicate also known as calcium orthosilicate (Ca2SiO4 or 2CaO.SiO2) is prepared in an electric oven from silica, lime, and reducing carbonated agents, and it is useful as a steel-deoxidizing agent.
It is also used as an alloying agent for copper, aluminium, beryllium, lead and magnesium alloys, and used as a “getter” for residual gases in vacuum tubes, etc.
Calcium compounds are widely used, where vast deposits in limestone (calcium carbonate, CaCO3) used directly as a building stone and indirectly for cement.
When limestone is heated in kilns (furnace) it gives off carbon dioxide (CO2) gas leaving behind quicklime (Burnt lime or calcium oxide, CaO), which reacts vigorously with water, and give slaked lime (calcium hydroxide, Ca(OH)2).
Slaked lime (Ca(OH)2) is used to make cement, as a soil conditioner, and in water treatment to reduce acidity, and in the chemical industries, It is also used in steel making process to remove impurities from the molten iron ore.
When calcium hydroxide mixed with sand, it takes up carbon dioxide (CO2) from the air and hardens as lime plaster.
Gypsum is also known as calcium sulfate (CaSO4) is used by builders as a plaster and by nurses for setting bones, as ‘plaster of Paris’.
Calcium carbide (CaC2) is produces when heating up a mixture of carbon & lime at 3000 ºC in an electric oven and it is an acetylate which produces acetylene (C2H2) by hydrolysis (chemical breakdown of a compound due to reaction with water).
The pure calcium carbonate (CaC2) occurs in two crystalline forms; Calcite in hexagonal shaped, and Aragonite in rhombohedric shaped.
It is very little soluble in water, but quite soluble if the water contains dissolved carbon dioxide (CO2), and forms calcium bicarbonate.
Calcium hypochlorite (whitening powder, Ca(ClO)2) is produced in the industry when passing chlorine through a lime solution, and has been used as a whitening agent, and as water purifier (bleaching powder, chlorine powder).
Calcium cyanamide (Ca(CN)2) is also commercially known as nitrolime, It is the calcium salt of the cyanamide anion, where this chemical is used as fertilizer (helps plants to reach high yields).
Biological role of Calcium
It has non-toxicity, even It is very essential in large quantities for all living things, particularly for the growth of healthy leaves, bones, teeth, and seashells.
Calcium phosphate (Ca3(PO4)2) is the main component of bone, and the average human contains about 1 KG (kilogram) of calcium.
Pregnant women and Children are encouraged to eat foods rich in calcium, such as milk and dairy products, green leafy vegetables, fish, and nuts & seeds.
From the IOM (U.S. Institute of Medicine), calcium for people ages 9–18 years are not supposed to exceed 3,000 mg/day, for ages 19–50 not to exceed 2,500 mg/day, for ages 51 and older, not to exceed 2,000 mg/day.
Abundance of Calcium
Calcium is the 5th most abundant metal in the Earth’s crust, and it is always found combined (merged with others) in nature.
it occurs abundantly as Limestone (calcium carbonate, CaCO3), Fluorite (calcium fluoride, CaF₂), Gypsum (calcium sulfate, CaSO4·2H2O), and Apatite (calcium chloro- or fluoro-phosphate, Ca5(PO4)3(F,Cl,OH)).
Hard water contains dissolved calcium bicarbonate (Ca(HCO3)2), and when this filters through the ground and reaches a cave, it precipitates out to form stalactites (icicle-shaped mineral deposit formation that hangs from the ceiling of a cave) and stalagmites (rock formation that rises from the floor of a cave).
Commercially, the metal is prepared by heating Lime with Aluminum in a vacuum.
Annual world wide production is around 50,000-70,000 tons.
0.007% (In Universe)
1.1% (In Meteorites)
0.007% (In Sun)
5% (In Earth’s Crust)
0.00042% (In Oceans)
1.4%(In Humans)




World’s Top 3 producers of Calcium
1) China
2) USA
3) INDIA
Calcium Price
Highly Pure (99.9%) metal price is around $7000-$8000 per KG (Kilogram) and Isotope (Calcium-48) Price is $250,000 per gram.
#Calcium



This is really helpful, thanks.