04 Be (Beryllium Element)

CONTENT INDEX
About Beryllium Element
Beryllium is a steel gray metal, and has many desirable properties. It is one of the Lightest metals with a high melting point. Its modulus of elasticity is about 1/3rd greater than that of steel.

It is relatively soft and has Low density, Excellent Thermal conductivity, and is Nonmagnetic. Due to these properties beryllium resists attack by concentrated Nitric acid, and at normal pressure and temperature it resist oxidation when exposes to air.
It has a high permeability to X-rays and when bombarded by Alpha particles, as from radium or polonium, neutrons are produced in the amount of about 30 neutrons/million alpha particles.
Identity
CAS Number: CAS7440-41-7
CID Number: CID5460467
DOT Hazard Class: 6.1
DOT Number: 1567
RTECS Number: RTECSDS1750000
Properties of Beryllium Element
Basic Properties of Beryllium
Pronunciation: ba-ri-Lee-am / bə-ri-liəm
Appearance: White-gray metallic
Mass Number: 9
Standard Atomic weight: 9.012 g/mol
Atomic number (Z): 4
Electrons: 4
Protons: 4
Neutrons: 5
Period: 2
Group: 2
Block: S
Element category: Alkaline earth metal
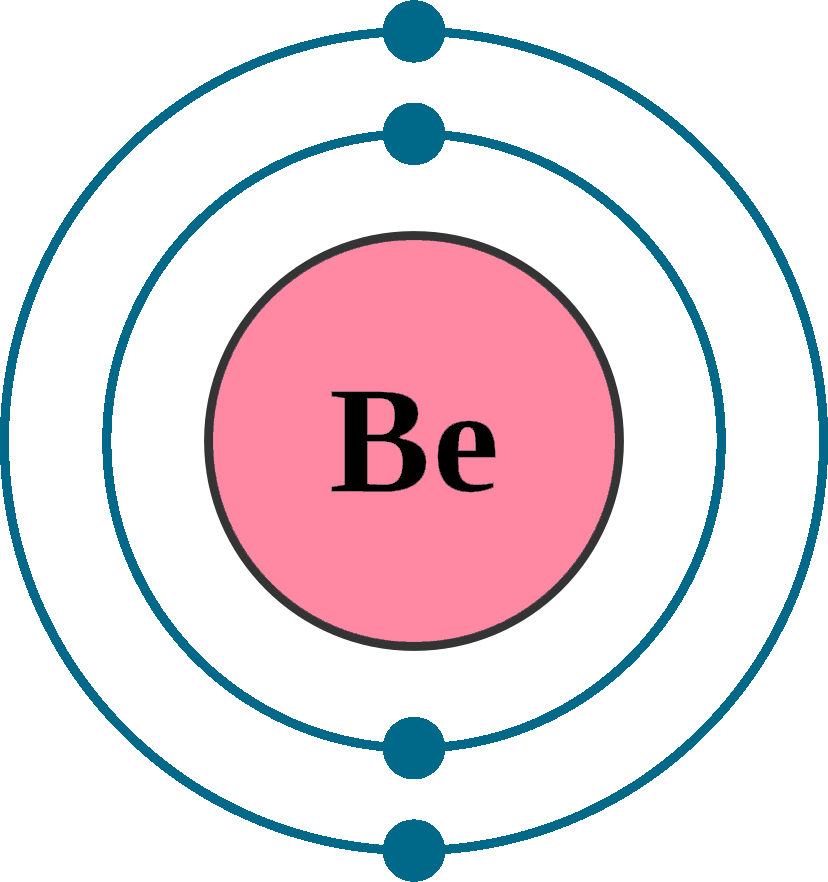
Electrons per shell: K2, L2
Electron configuration: 1s22s2
Thermal Properties of Beryllium
Phase: Solid
Melting point: 1560 K (1287 oC, 2349 oF)
Boiling point: 2742 K (2469 oC, 4476 oF)
Critical Temperature: 5202 K (4932 oC, 8909 oF)
Debye temperature: 1000 K (726.85 oC, 1340.33sx oF)
Fusion heat: 12.2 kJ/mol
Vaporization heat: 292 kJ/mol
Specific heat: 1820 J/(kg K)
Molar heat capacity: 16.442 J/(mol.K)
Thermal expansion: 11.3 μm/(m∙K)
Thermal conductivity: 200 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Beryllium
Electrical conductivity: 25 x106 S/m
a Electrical resistivity: 36 nΩ∙m
Electrical type: Conductor
Critical point (Superconducting point): 0.026 K (-273.12 oC, -459.62 oF)
Magnetic Properties of Beryllium
Magnetic type: Diamagnetic
Curie point: N/A
Magnetic susceptibility (xmol): -9.0×10-6 cm3/mol
Volume magnetic susceptibility: -0.00002328
Mass magnetic susceptibility: -12.6×10-9 m3/kg
Molar magnetic susceptibility: -0.1136×10-9 m3/mol
Physical Properties of Beryllium
Density: 1.85 g/cm3 (In solid) 1.69 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000048767 m3/mol
Young’s modulus: 287 GPa
Shear modulus: 132 GPa
Mohs Hardness: 5.5
Bulk modulus: 130 GPa
Poisson ratio: 0.032
Vicker hardness: 1670 MPa
Brinell hardness: 590-1320 MPa
Sound Speed: 12,890 m/s
Atomic Properties of Beryllium
Oxidation states: +1, +2
Valence Electrons: 2s2
Ion charge: Be2+
The ionization potential of an atom: 9.50 eV
Ionization energies: 1st: 899.5 kJ.mol 2nd: 1757.1 kJ/mol 3rd: 14,848.5 kJ/mol
Ionic radius: 35 pm
Atomic radius: 112 pm (empirical)
Van der Waals: 153 Pm
Covalent radius: 93±3 pm
Filling Orbital: 2s2
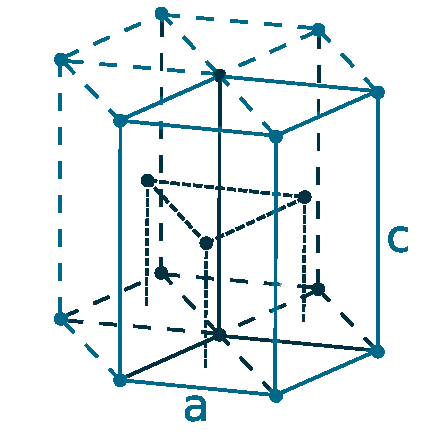
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 228.6, 228.6, 358.4 pm
Grid parameters: a=2.286 Å c=3.584 Å
Attitude c/a: 1.567
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Beryllium
Electronegativity: 1.57 (pauling scale)
Valence: 2
Electron affinity: 0 kJ/mol
Nuclear Properties of Beryllium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 0.0092
Neutron Mass Absorption: 0.00003
Isotopes: 7Be9Be10Be
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 7Be | Trace | – | 53.12 Day |
| 9Be | 100 | 9.012 | Stable |
| 10Be | Trace | – | 1.39×106 y |
Chemical Reactions of Beryllium Element
Reaction with Air
Beryllium is passivated by oxygen, and forming a BeO surface. Normally beryllium cannot be oxidized, but powdered beryllium can be burn in air, and forming Beryllium Oxide (BeO), and Beryllium Nitride (Be3N2).
2 Be (s) + O2 (g) → 2 BeO (s)
3 Be (s) + N2 (g) → Be3N2 (s)
Reaction with Water
Beryllium doesn’t react with water or steam, even if the metal is heated to red hot.
Reaction with Halogens
The metal reacts with chlorine and bromine, and forming the corresponding Beryllium (II) dihalides.
Be (s) + Cl2 (g) → BeCl2 (s)
Be (s) + Br2 (g) → BeBr2 (s)
Reaction with Acids
Beryllium is passivated by oxygen, and forming a beryllium oxide (BeO) which is resistant to acid. But on a fresh surface (without protective layer), beryllium dissolves readily in dilute acid such as Hydrochloric acid (HCl), Sulphuric acid (H2SO4), and Nitric acid (HNO3), and forming Beryllium (II) ions and hydrogen gas (H2).
Ba (s) + 2 H+ (aq) → Ba2+ (aq) + H2 (g)
Beryllium History
Naming: From the mineral Beryl.
Discovery: Nicolas Louis Vauquelin (1798) in Paris (France)
First isolation: Friedrich Wöhler & Antoine Bussy (1828)
Beryllium Uses
Beryllium is used as an alloying agent in the production of beryllium-copper (BeCu, 1-3% Be) or beryllium-nickel (BeNi), which is extensively used to make Gyroscopes, Electrical contacts, Springs, Spot-welding Electrodes, and Non-sparking tools.
Because of Its electrical and thermal conductivity, high strength and hardness, good resistance, non-magnetic properties, It also used in the Defense and Aerospace industries as a structural material for high-speed aircraft, Missiles, Spacecraft, and Communication Satellites.
Other uses are in brake discs, windshield frame, support beams, and other structural components of the space shuttle.
Beryllium is relatively transparent to X-rays, so ultra-thin Beryllium-foil is finding use in X-ray lithography for the reproduction of micro-miniature integrated circuits.
Be (Beryllium) is used in Nuclear reactors as a reflector or moderator of neutrons.
It is also used in Computer parts, and instruments where stiffness, lightness, and dimensional stability are required.
The oxide has a very high melting point, so it is also used in nuclear work and ceramic applications.
Biological role of Beryllium
Beryllium is not the crucial element for human, in fact the element and its compounds are Toxic & Carcinogenic and it should be handled with the greatest care. Even it should not be tasted to verify the sweetish nature of beryllium (as did early experimenters).
If humans breath or inhale the dust or fumes of beryllium, It can damage the Lungs, cause pneumonia, and can lead to an incurable (seriously ill) inflammation of the lungs called Berylliosis (chronic beryllium disease, CBD).
beryllium can also increase the chances of cancer development and DNA damage.
That’s why the metal, its alloys, and its salts should be handled with proper safeguards.
Be (Beryllium) occurs naturally in the environment in small amounts, while Humans add beryllium in the environment through production of metal and combustion of coal and oil.
Beryllium Element Sources
Beryllium Element is found in about 30 different mineral species, the most important of which are Bertrandite (beryllium sorosilicate hydroxide, Be4(Si2O7)(OH)2), Beryl (beryllium aluminium cyclosilicate, Be3Al2Si6O18), Chrysoberyl (BeAl2O4), and Phenakite (Be2SiO4).
Emerald and Aquamarine are the precious forms of beryl.



Bertrandite and Beryl are the most important commercial sources of the element. Most of the metal is prepared by reducing beryllium fluoride with magnesium metal.
The Beryllium content on Earth crust is about 2.6 ppm, in soil 6 ppm.
Total World wide Reserve of Beryllium ore is around 500,000 tonnes.
1×10-7% (In Universe)
2.9×10-6% (In Meteorites)
1×10-8% (In Sun)
0.00019% (In Earth’s Crust)
6×10-11% (In Oceans)
4×10-8% (In Humans)
World’s Top 3 producers of Beryllium
1) USA
2) China
3) Mozambique
World’s Top 3 Reserve holders of Beryllium
1) Unknown (likely USA)
Beryllium Element Price
Pure (99.95%) metal price is around $700-$800 per 100 Gram ($7-$8 per Gram).
Database of Beryllium Element
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
→ Electrons (ESTAR)
→ Helium Ions (ASTAR)
→ Protons (PSTAR)
Nuclear Physics Data
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements


