Periodic table groups and periods – As you move from the light elements to the heavy elements, the ordinary properties are prepared so that you can easily see similarities between elements. You keep periodically coming across the same properties, that’s why it’s called the periodic table. The periodic table is arranged in periods and groups, going from the light elements at the top, to the heavy elements at the bottom. The rows that go from left to right are periods.
Periodic Table Periods:
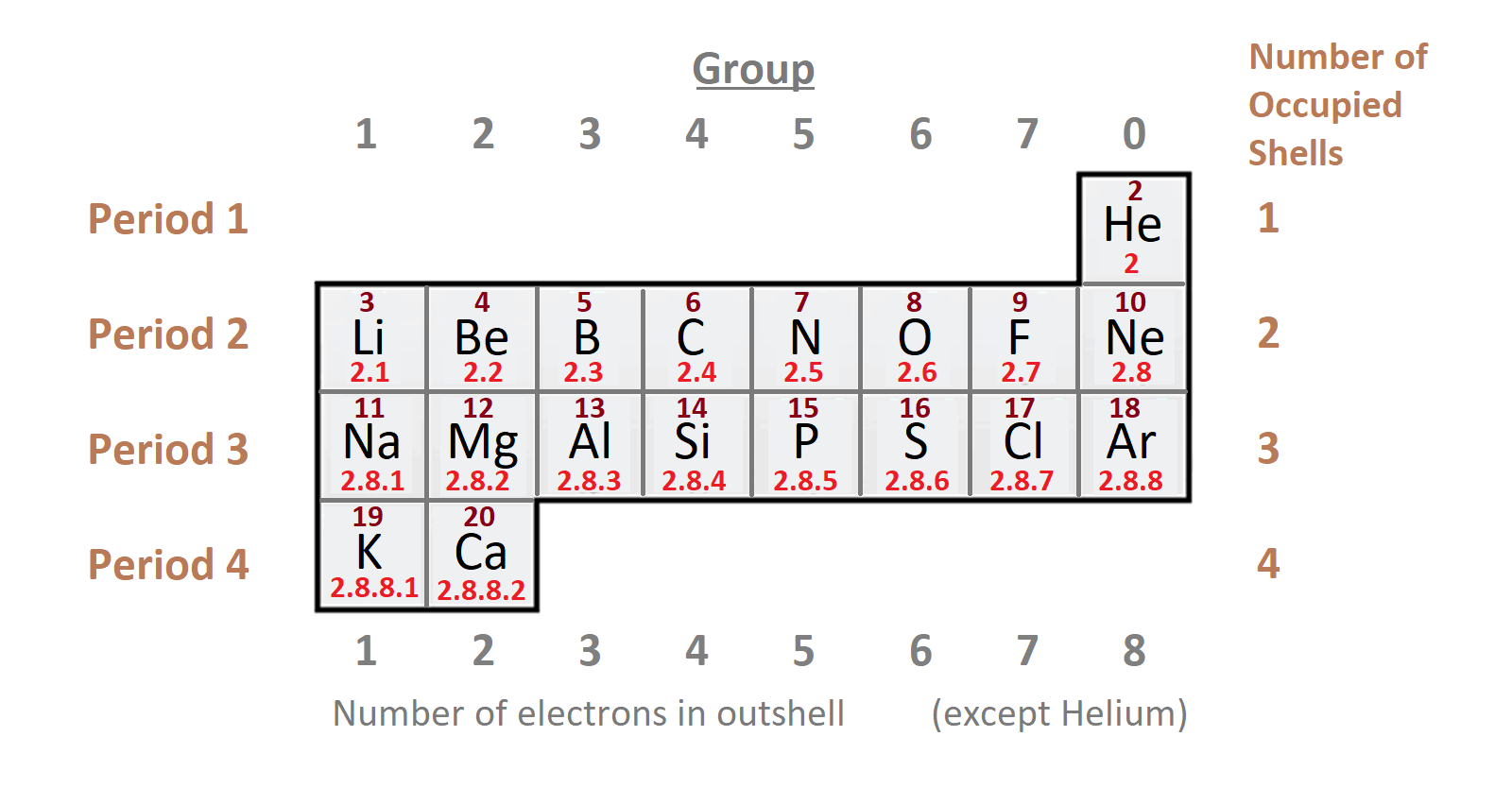
The horizontal rows are called periods. There are seven periods in the long form of the periodic table.
1st period: (H to He) it consists of two elements. It is the shortest period. We can find out the total number of shells in the atom of the element from the period number. For instance: if any element is in fifth period, it means that element have 5 shells.
2nd period: It is starts from lithium and end to neon (3Li to 10Ne).
3rd period: Third period is move from sodium to argon (11Na to 18Ar).
They both contain 8 elements each are called short periods.
4th period: It move from (19K to 36Kr). And, total 18 elements are present in this period.
5th period: It goes from (37Rb to 54Xe). It also has 18 elements.
They both contain 18 elements each are called long periods.
6th period: (55Cs to 86Rn) consists of 32 elements and it is known as the longest period.
7th period: seventh period is starting with 87Fr and consists of 32 elements.
Period 1st: H, He (they do not follow the octet rule).
Period 2nd: Li, Be, B, C, N, O, F, Ne (including s and p orbitals)
Period 3rd: Na, Mg, Al, Si, P, S, Cl, Ar (all with at least one isotope)
Period 4th: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements)
Period 5th: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Te, I, Xe (number of elements similar to period 4th same overall structure, included the first element is exclusively radioactive element, Tc).
Period 6th: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f block elements).
Period 7th: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, NO, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (All elements are radioactive. And, they contain the heaviest natural elements).
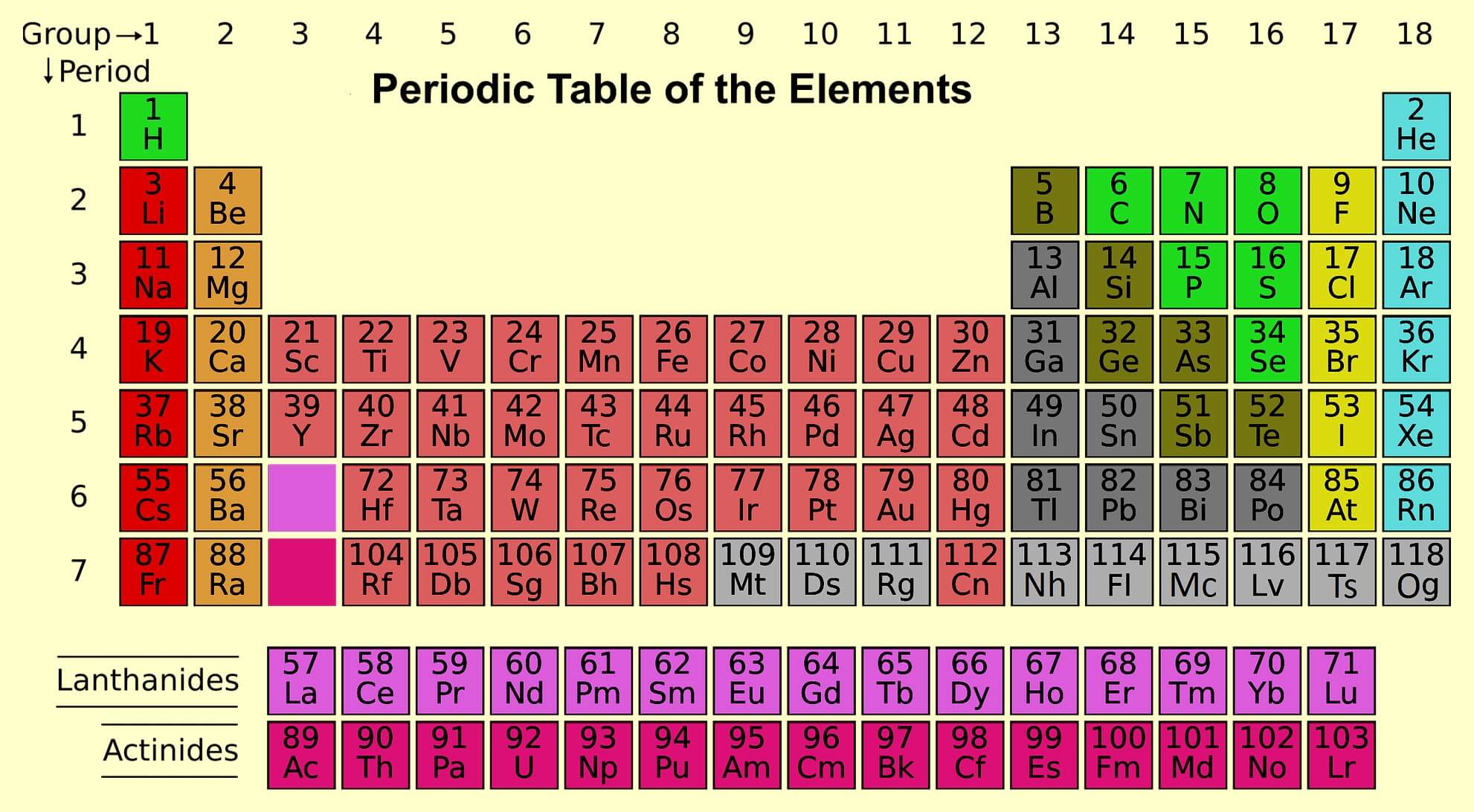
Periodic Table Groups
- 18 vertical columns are called groups.
- Elements that belong to a specific group are called a family and are usually named using the first number.
- Two different numbering systems are often used to represent groups, and you should be familiar with both.
- The traditional system used in the United States involves the use of the letters A and B.
- The first two groups are 1A and 2A, while the last six groups are 3A through 8A.
- Middle B groups are used in their title. Unfortunately, there is a slightly different system in Europe.
- To avoid any confusion, the International Union of Pure and Applied Chemistry (IUPAC) has decided to simplify the numerical system from 1 to 18 from left to right.
- Many time tables show two systems at once. As part of this, some groups share a common name as shown below.
Elements of group 1st are called alkali metals. (Except hydrogen).
Elements of group 2nd: The elements of the second group are called alkaline earth metals.
Elements of group 3rd to 12th are called as transition elements or d block elements.
Elements of group 13th and 14th: Group 13th elements are known as boron family, while group 14th elements are called as carbon family.
Elements of group 15: The elements of 15th group are called pnictogens. It means suffocation of the system due to toxicity. Pnictogens is made up by pnicto + gens, in which pnicto means poison and gens means formation.
Elements of group 16 are called chalcogens. It is also known as the oxygen family. They found in the form of ore that’s why they are called as chalcogens.
Elements of group 17 are called halogens. They form salt and found in the form of salt that’s why they are called as halogens. Because meaning of halo is salt.
Elements of group 18 are called as noble gas or aerogenes. Cause they do not chemical reaction at normal temperature. They are also called as inert gas.
| Groups | Family |
| Group 1 | lithium family |
| Group 2 | beryllium family |
| Group 3 | scandium family |
| Group 4 | titanium family |
| Group 5 | vanadium family |
| Group 6 | chromium family |
| Group 7 | manganese family |
| Group 8 | iron family |
| Group 9 | cobalt family |
| Group 10 | nickel family |
| Group 11 | copper family |
| Group 12 | zinc family |
| Group 13 | boron family |
| Group 14 | carbon family |
| Group 15 | nitrogen family (pnictogens) |
| Group 16 | oxygen family (chalcogens) |
| Group 17 | fluorine family (halogens) |
| Group 18 | helium family (noble gases) |
Elements within a group have the same number of valence electrons. The number of valence electrons depends on the octet law. For example, Group 1st elements have 1 electron capacity, Group 3-12 elements have a variable number of valence electrons, and Group 17th elements have 7 valence electrons. The lanthanides and actinides listed in the main table below all fall into Group 3rd.
There are 18 element groups. The elements of a group have chemical and physical properties in common. For example, Group 1 elements are all soft and reactive metals. Group 17 elements are highly reactive and non-ferrous metals.
Magic number: 2,8,8,18,18,32,32 are magic numbers.
So now I hope you understand about periods and groups in periodic table. How elements are arranged in periodic table, how they divided into these two categories groups and periods.