80 Hg (Mercury)

It is a heavy, silvery-white metal, and sometimes called it quicksilver.
Mercury is the only metal that is in liquid form at normal temperatures, and It is a poor conductor of heat, but a fair conductor of electricity.
Mercury is a poison, which is readily absorbed through the gastro-intestinal tract, respiratory tract, or through unbroken skin.
At 20°C, air saturated with mercury vapour contains a concentration that exceeds the toxicity limits, and its toxicity is increased with increasing the temperature.

Identity
CAS Number: CAS7439-97-6
CID Number: CID23931
DOT Hazard Class: 8
DOT Number: 2809
RTECS Number: RTECSOV4550000
CONTENT INDEX
Basic Properties of Mercury
Pronunciation: mur-kyuh-ree
Appearance: silvery
Mass Number: 201
Standard Atomic weight: 200.592 g/mol
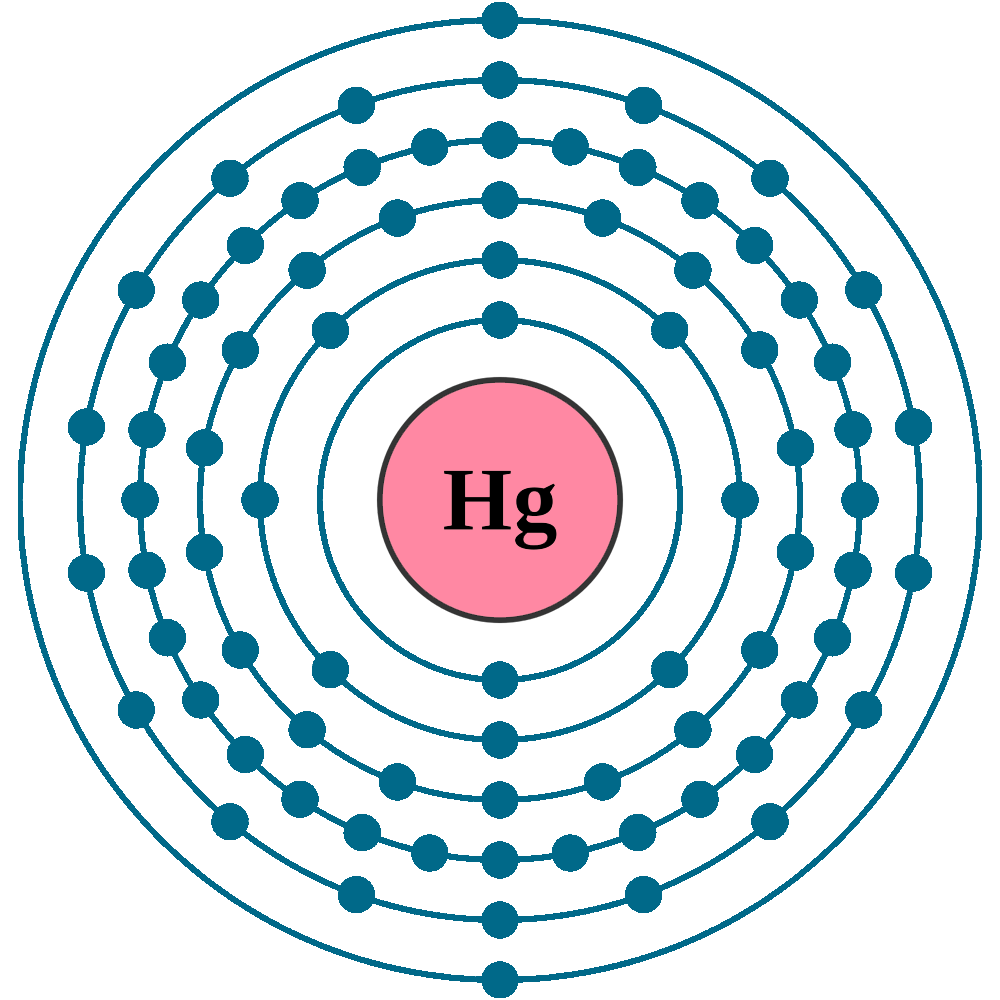
Atomic number (Z): 80
Electrons: 80
Protons: 80
Neutrons: 121
Period: 6
Group: 12
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N32, O18, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s2
Thermal Properties of Mercury
Phase: Liquid
Melting point: 234.32 K (-38.82 oC, -37.89 oF)
Boiling point: 629.88 K (356.73 oC, 674.11 oF)
Debye temperature: 100 K (-173.15 oC, -279.67 oF)
Triple point temperature: 234.31 K (-38.84 oC, -37.912 oF)
Triple point pressure: 1.65×10-7 KPa
Critical point temperature: 1750 K (1476.85 oC, 2690.33 oF)
Critical point pressure: 172 MPa
Fusion heat: 2.29 kJ/mol
Vaporization heat: 59.10 kJ/mol
Specific heat: 139 J/(kg K)
Molar heat capacity: 27.984 J/(mol.K)
Thermal expansion: 60.4 μm/(m∙K)
Thermal conductivity: 8.30 W/(m∙K)
Electrical properties of Mercury
Electrical conductivity: 1×106 S/m
A Electrical resistivity: 961 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 4.154 K (-269 oC, -452.2 oF)
Magnetic Properties of Mercury
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -33.4×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000284
Mass magnetic susceptibility: -2.1×10-9 m3/kg
Molar magnetic susceptibility: -0.421×10-9 m3/mol
Physical Properties of Mercury
Density: 13.534 g/cm3
Molar volume: 0.0000148213 m3/mol
Bulk modulus: 25 GPa
Refractive index: 1.000933
Sound Speed: 1451 m/s
Atomic Properties of Mercury
Oxidation states: 2, 1, -2
Valence Electrons: 6s2
Ion charge: Hg2+
Ionization potential of an atom: 10.39
Ionization energies: 1st: 1007 kJ.mol 2nd: 1810 kJ/mol 3rd: 3300 kJ/mol
Ionic radius: 102 pm
Atomic radius: 151 pm (empirical)
Van der Waals: 155 Pm
Covalent radius: 132±5 pm
Filling Orbital: 5d10
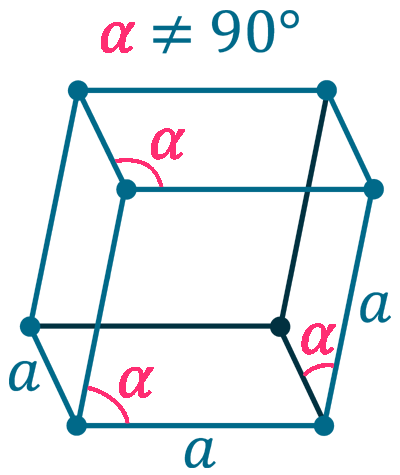
Crystal structure: Rhombohedral
Grid parameters: a(hex)=3.464 Å c(hex)=6.708 Å
Attitude c/a: 1.94
Space Group Name: R_3m
Space Group Number: 166
Reactivity of Mercury
Electronegativity: 2.00 (pauling scale)
Valence: +2
Electron affinity: 0 kJ/mol
Nuclear Properties of Mercury
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 1S0
Neutron cross section (Brans): 374
Neutron Mass Absorption: 0.063
Isotopes: 194Hg 195Hg 196Hg 197Hg 198Hg 199Hg 200Hg 201Hg 202Hg 203Hg 204Hg
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 194Hg | Syn | – | 444 y |
| 195Hg | Syn | – | 9.9 h |
| 196Hg | 0.15 | 195.964 | Stable |
| 197Hg | Syn | – | 64.14 h |
| 198Hg | 10.04 | 197.965 | Stable |
| 199Hg | 16.94 | 198.967 | Stable |
| 200Hg | 23.14 | 199.968 | Stable |
| 201Hg | 13.17 | 200.971 | Stable |
| 202Hg | 29.74 | 201.970 | Stable |
| 203Hg | Syn | – | 46.61 d |
| 204Hg | 6.82 | 203.972 | Stable |
Chemical Reactions of Mercury
It reacts in air at 350 oC, and forming:
2 Hg (l) + O2 (g) → 2 HgO [red] (mercury (II) oxide)
Doesn’t react with water.
The metal reacts with all halogens, and forms mercury dihalides:
Hg (l) + F2 (g) → HgF2 (s) [white] (mercury (II) fluoride)
Hg (l) + Cl2 (g) → HgCl2 (s) [white] (mercury (II) chloride)
Hg (l) + Br2 (g) → HgBr2 (s) [white] (mercury (II) bromide)
Hg (l) + I2 (g) → HgI2 (s) [white] (mercury (II) iodide)
Hg(l) is precipitated by Cl– ions, and forming:
Hg22+ (aq) + 2 Cl– (aq) → Hg2Cl2 (s) [white]
Hg(II) is precipitated by I– ions, and forming:
Hg2+ (aq) + 2 I– (aq) → HgI2 (s) [red]
The precipitate is dissolved in excess I–, and forming almost colourless tetraiodo mercurium complex:
HgI2 (s) [red] + 2 I– (aq) → [HgI4]2- (aq)
The metal doesn’t react with non-oxidizing acids, but does react with concentrated nitric (HNO3) or suphuric acid (H2SO4), and forming mercury (II) compounds together with nitrogen or sulphur oxides.
Mercury dissolves slowly in dilute nitric acid (HNO3), and forms mercury (I) nitrate, mercurousnitrate (Hg2(NO3)2).
2 Hg (l) + 2 HNO3 (aq) → Hg2(NO3)2 (aq) + H2(g)
The metal doesn’t react with alkalis under normal conditions.
Hg (I) as Hg22+ reacts with hydroxide, and forming:
Hg22+ (aq) + 2 OH– (aq) → Hg2O (s) [black]
The precipitate is split up in HgO & Hg upon heating:
Hg2O (s) → HgO (s) + Hg (l)
Hg(II) reacts with hydroxide OH– under cold conditions, and forming a yellow precipitate of HgO:
Hg2+ (aq) + 2 OH– (aq) → HgO (s) [yellow] + H2O (l)
The precipitate turns red, upon heating.
Production
Hg is extracted by heating cinnabar in air & condensing the vapour:
HgS + O2 → Hg + SO2
Mercury History
Naming: Symbol Hg taken from mercury’s Latin name: Hydrargyrum, which comes from the Greek word “hydrargyros” (“hydor” for water & “argyros” for silver).
Discovery: Ancient Chinese and Indians (before 2000 BCE)
Mercury Uses
It is a heavy liquid metal, and because of its highly toxicity,many uses are being phased out or in under review.
Now mercury is mainly used as catalysts in the chemical industry, and It is also used in some electrical switches & rectifiers.
Mercury metal is use in Industry as a liquid electrode in the manufacture of chlorine & sodium hydroxide (Cl2 & Caustic soda, NaOH) by electrolysis of brine (NaCl water).
Because of its high density the metal is used in manometers & barometers, extensively used in thermometers, and extensively used in thermometers, and due to its high rate of thermal expansion, it is fairly constant over a wide range of temperature.
Mercury forms alloys (called amalgams) with other metals such as silver, gold, & tin, where amalgams with gold is useful in recovering gold from gold ores, and with Mercury is used in dental fillings.
Vermilion (Mercuric sulfide, HgS) is a high-grade, bright-red paint pigment, which is very toxic, so it is used with great care.
Calomel (mercurous chloride, Hg2Cl2) is used as a purgative in medicine , and as a standard in electrochemical measurements.
Mercuric oxide (mercury oxide, HgO) is used in skin ointments.
Mercury fulminate (Hg(CNO)2) is a primary explosive, so it is used as a detonator.
Red mercury can used in the creation of nuclear bombs, and variety of unrelated wepons systems.
Mercuric sulphate (mercury sulfate, HgSO4) is used as a catalyst in organic chemistry.
Mercuric chloride (corrosive sublimate, HgCl2) is a highly toxic compound, which is used as an insecticide, in rat poison, & as a disinfectant.
The metal is also used in making mercury-vapor lamps, advertising signs, Rockets, Mesiles, military prospecting areas etc..
Biological role of Mercury
It is present in every living thing & widespread in the environment, even every food we eat contains a little amount of mercury.
We intake daily is less than 0.01 mg (milligrams) & about 0.3 grams in a lifetime, but in much higher doses it will be toxic and one form of mercury (methylmercury, [CH3Hg]+) is particularly very dangerous.
Once mercury reaches to the surface of waters or soils, microorganisms can convert it to methylmercury.
Methylmercury can accumulate (gather together) in the flesh of fish & be eaten by people, which makes them ill (damage to intestines, stomach disruption, kidneys damage, reproductive failure and DNA alteration etc..)
It acts as a cumulative poison (means, once it enters into the body, it accumulates in such areas: Blood, Liver, Kidneys, & Brain) and dangerous levels are readily attained in air.
In form of vapour It is highly dangerous, because it easily enters in the body.
It is important that mercury should be handled with care, where containers of mercury should be securely covered & spillage should be avoided, and If it is necessary to heat mercury or its compounds, it should be done in a well-ventilated hood (head covering).
Abundance of Mercury
Mercury is an rare element, which is mostly found in nature in form of combined state, and it can be found as droplets in cinnabar ores (mercury sulfide, HgS).
The metal is recovered by heating cinnabar in a current of air & condensing the vapour.
Annual world wide production is around 11,000 tons.
1×10-7% (In Universe)
0.000025% (In Meteorites)
2×10-6% (In Sun)
6.7×10-6% (In Earth’s Crust)
5×10-9% (In Oceans)

World’s Top 3 producers of Mercury
1) China
2) Kyrgzstan
3) Chile
World’s Top 3 Reserve holders of Mercury
1) Mexico
2) China
3) Kyrgyzstan
Mercury Price
Pure (99.99%) metal price is around $400-$500 per KG (KiloGram)
#mercury