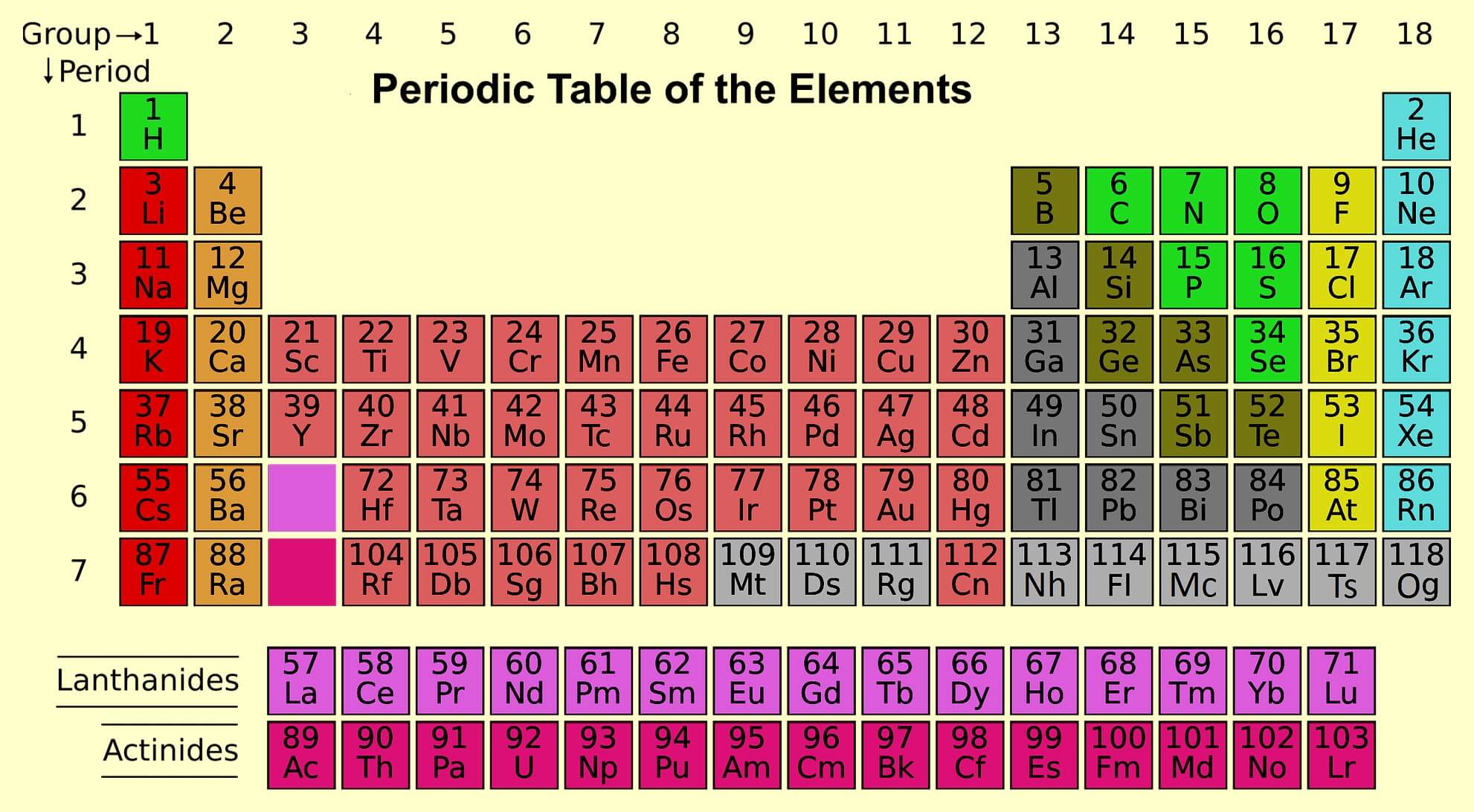
Long Form Modern & Mendeleev Periodic Table of Elements
CONTENT INDEX
History of Periodic Table
In 1800, only 30 elements were discovered that time. According to time, this invention was increased and 56 elements were found in 1865. Then in 1869, 69 elements were found that time. And in 2019, 118 elements were found. As of now, 118 elements are present in periodic table. So, the study of these all elements was very typical. Therefore, scientists decided that it would be easier to study about these all elements if they would divide them into groups according to their properties. And groups of these elements are known as periodic table.
What is the Periodic Table?
Sorting the elements by their properties, so that elements with similar properties are grouped in a table. This table is known as periodic table.
Development of periodic table
1. Antoine Lavoisier classification: He is known as “Father of Chemistry“. He divided elements in two categories which are metals and nonmetals, But he did not explain about metalloids.
2. Prout’s hypothesis: According to him all the elements are made up of hydrogen. It means that atomic weight of an element is equal to atomic weight of hydrogen. Which is not possible, that’s why there were some limitations.
3. Dobereiner’s triad: In 1817, approximately 30 elements were known that time. He arranged elements with similar properties in a group of three known as triads. And he found that the average atomic mass of the element is roughly or exactly to the middle element. For example: atomic mass of Li (lithium) is 6.9, and atomic mass of K is 39. So average atomic mass of these both elements will be 22.9. which is the atomic mass of Na (sodium). And here Na is the middle element.
Limitations: this concept was not accepted by all the elements at that time. For example. Atomic mass of N (nitrogen) is 14 and atomic mass of As (arsenic) is 74.5. So, the average atomic mass of these elements will be 44.25. while atomic mass of middle element is 31, which is the atomic mass of P (phosphorous). that’s why this concept was not applicable for all.
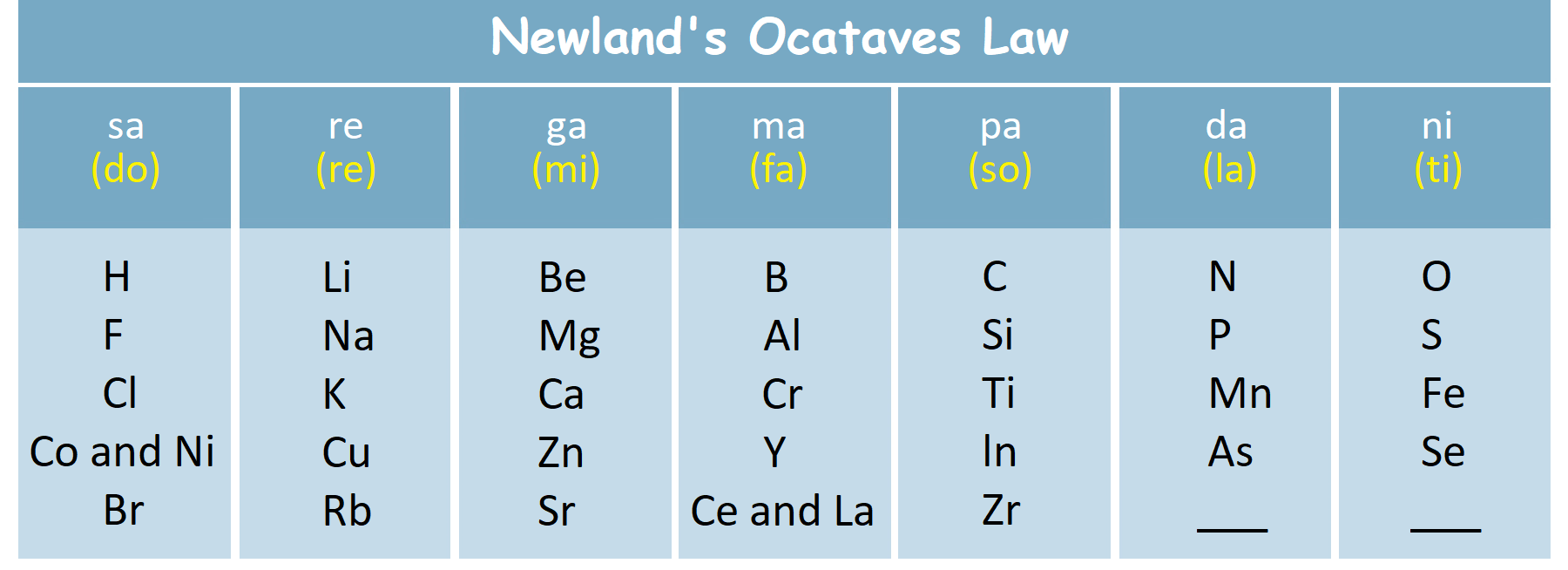
4. Newland’s Octaves Law: In 1865, A British chemist J.A.R. Newlands were found 56 elements. He arranged elements horizontally according to increasing atomic mass and found that the element with similar property get repeated itself at the 8th position just like the musical scale, Like Sa Re Ga Ma Pa Dha Ni Sa.
Limitations: this concept holds good only up to lighter elements. according to him no new element would be discovered in future. After the discovery of noble gases, it was the 9th element which get repeated.
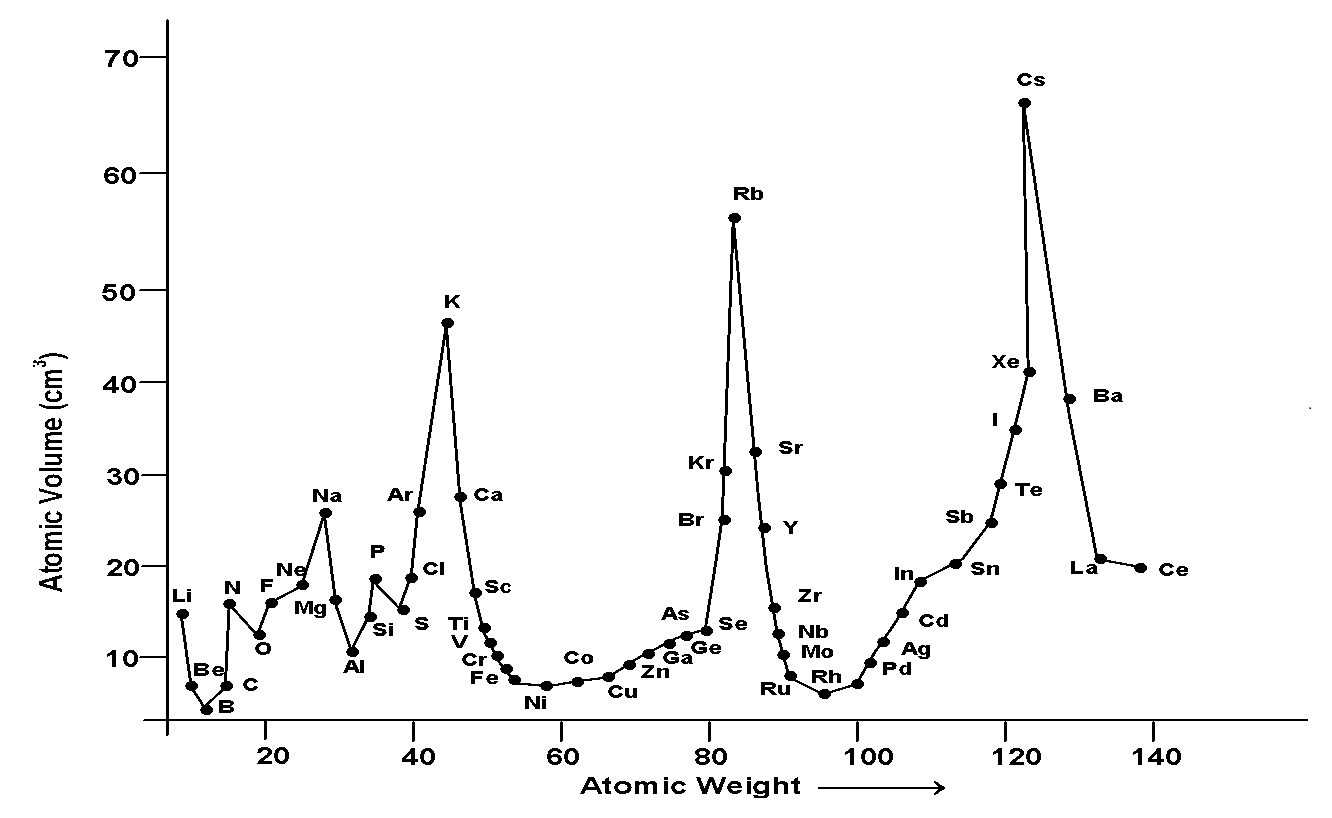
5. Lothar Meyer classification of elements: 1869, he plotted a graph of atomic volume verses atomic mass.
Features:
1) The elements (alkali metals) Li, Na, K, Rb, Cs occupied the peak position in graph.
2) The bottom position was occupied by transition metals and metalloid.
3) Ascending position were occupied by halogens (F, Cl, Br, I).
4) Descending positions were occupied by alkaline earth metals (Be, Mg, Ca, Sr, Ba).
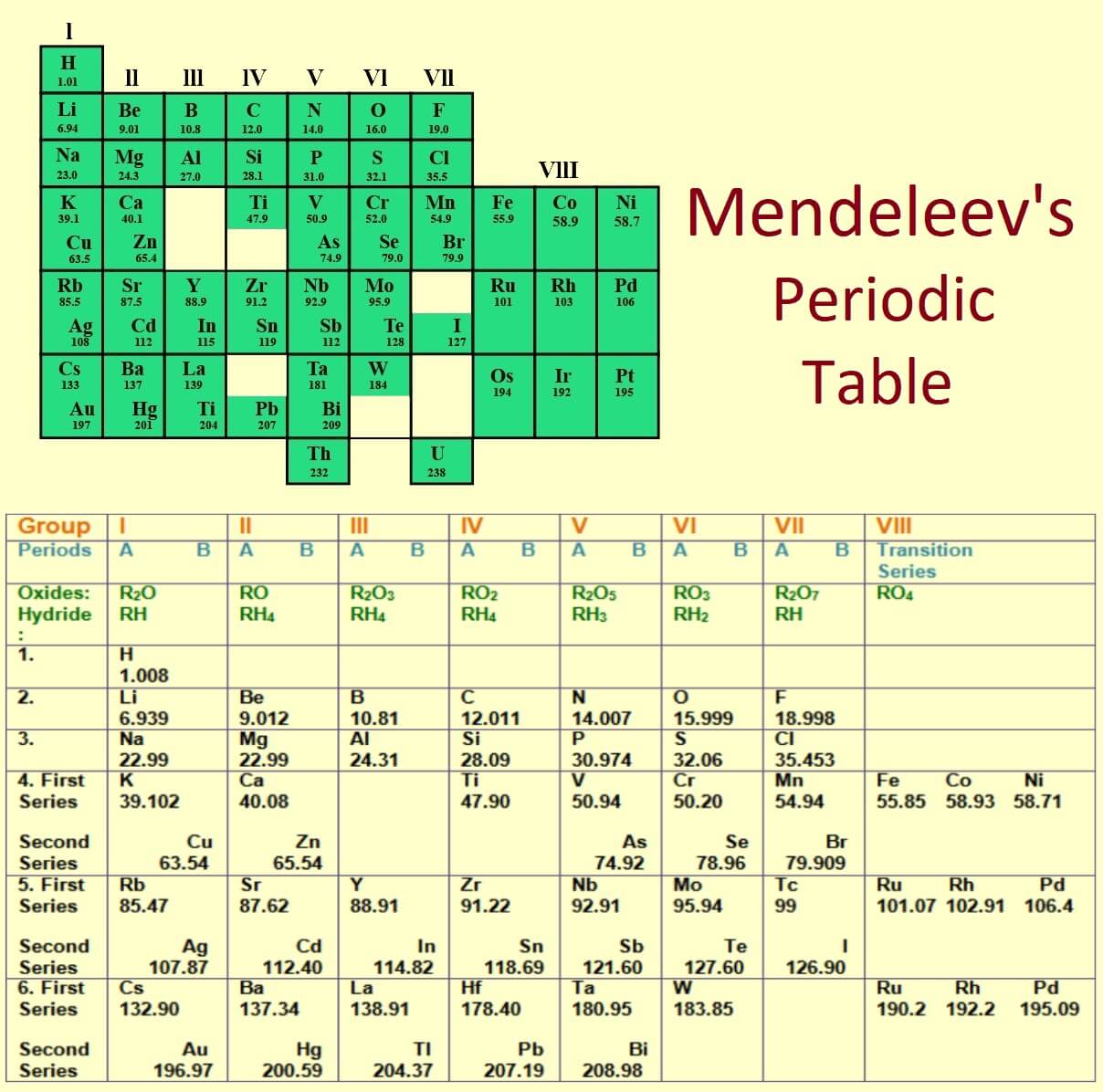
Mendeleev’s Periodic Table
- This periodic table is given by Dmitri Mendeleev in 1869.
- 63 elements were known at that time.
- He was the first scientist who made this periodic table in very systematic way.
- He got noble prize for his work in 1906.
The Real Breakthrough in Periodic Table: This concept was based on increasing atomic mass of elements. Mendeleev published the first periodic table of elements in 1869. Mendeleev in his periodic table, As the atomic mass increases, the elements are arranged in rows. In a series the elements with low atomic mass were on left side. And, the elements in the column have similar properties.
They found two types of columns in which one is horizontal column and second is vertical column. and he gave name these horizontal column and vertical column as period or group respectively. These groups and periods are 8 or 7 in numbers. Each group was subdivided into subgroup A and B except 8th group. This 8th group are of transition metals.
Merits of Mendeleev Periodic Table
- Systematic study of element
- Correction of some doubtful atomic mass. For example – Be, U, In.
- He Predicted some new elements and named them after Sanskrit word Eka (next to). for example – eka boron-Sc, eka aluminum-Ga, eka silicon-Ge, Eka manganese-Tc.
- Even after the discovery of noble gases, Mendeleev periodic table was not disturbed.
- A zero group (valency=0) was created at the extreme right of the periodic table and noble gas were placed there.
Limitations of Mendeleev periodic table:
- Position of hydrogen.
- Position of isotopes (same atomic number but different mass number).
- Anomalous position of some pair of elements. (It is Based on increasing atomic mass) For example – atomic mass of Co (Cobalt) is 58.9 and atomic mass of Ni (Nickel) is 58.7. So according to their atomic mass Co should come first in periodic table, while Ni is coming first in periodic table. It is also applicable on Ar (Argon) and K (Potassium).
- No resemblance in the element placed in same group.
- Uncertainty in the prediction of new elements.
- This concept was based on atomic mass.
Modern periodic table:
- This periodic table is given by henry Moseley in 1913.
- H. Moseley was an English physicist.
- He measured the frequency of x-rays emitted by metals on bombarding high-speed electron on them.
- He plotted a graph of v versus atomic number.
- Moseley found a straight-line graph which was not found in case of atomic mass.
- He found that atomic number is more fundamental than atomic mass.
- Mendeleev periodic table was modified into modern periodic law. According to this law, the physical and chemical properties of elements are periodic function of their atomic number. If the elements are placed according to increasing atomic number than the element with similar property get repeated itself after a regular interval of time.
Long Form of Periodic Table
Latest periodic table is known as long form of modern periodic table. It was given by Rang and Werner. This is also known as Bohr table as it was based on Bohr scheme. In this periodic table group numbers were replaced by 1-18.
Groups
The vertical columns in periodic table are called group (8 groups).
1st group – alkali metals
2nd group – alkaline earth metals, d block elements are known as transition metals or elements.
15th group – this group are known as pnictogen.
16th group – this group are known as chalcogen.
17th group – they are known as halogen. And they are sea salt producer.
18th group – this group are of noble gas elements.
Periods
The horizontal columns are called period (7 periods).
Lanthanoids and actinoids comes in 6th or 7th periods.
Magic number – 2,8,8,18.18,32,32 these are magic numbers. 1st period-2, 2nd period-8, 3rd period-8, 4th period-18, 5th period-18, 6th period-32, 7th period-32.
Periodic table are divided into four Blocks which are following:
S block – 1-2 group
P block – 13-18groups
D block – 3-12 groups
F block –3rd group
What are Representative Elements?
Elements of s and p block are known as representative elements except noble gas.