Lanthanides On The Periodic Table
CONTENT INDEX
About Lanthanides
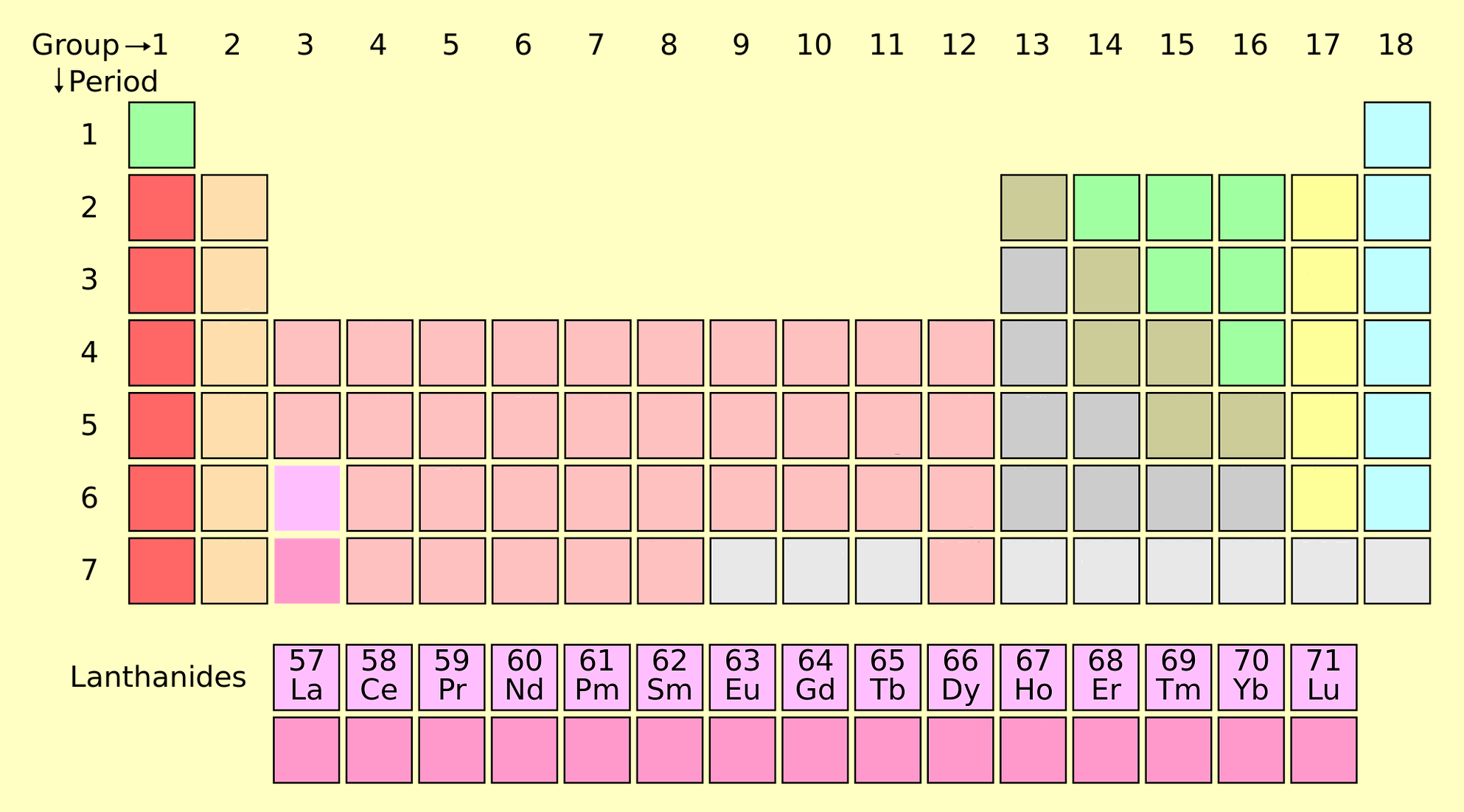
15 elements from Lanthanum (atomic number-57) to lutetium (atomic number-71) are called lanthanides. They include Lanthanum (La), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb), Lutetium (Lu).
- These are the 4f block elements of the periodic table.
- It is also known as first inner transition element.
- In the series of 15 elements in which first member is lanthanum that’s why they are called Lanthanide.
- They are also called as rare earth elements because first element lanthanum was found in very low concentration, so scientist was thought that time the abundance of other elements would also be very less. hence, they are known as rare earth elements.
Position: They are placed at the bottom of the periodic table. These are the group third and 6th period elements.
Occurrence: lanthanides are found in minerals. But the most important mineral is monazite mineral which is a black color sand.
Electronic configuration: The general electronic configuration of lanthanides is [Xe] 4f0-14 5d0-1 6s2. They are called 4f elements because they do not fill in 4f subshell completely.
Properties of Lanthanides
Physical Properties of Lanthanides
- Physical state: They are silvery white soft metals. Their hardness increase with increase in the atomic number, But samarium is exception here because they hard as steel.
- Melting and boiling point: They have high melting and boiling point. Their melting point varies from 1000K-1200K, But Samarium has exceptionally very high melting point and that is 1623K.
- Electrical and thermal conductivity: They are good conductor of heat and electricity.
- Density: They have high density due to greater mass.
- Color: These metals show color in solid state as well as the aqueous solution due to f-f transition.
La3+, Lu3+ = Colorless
Ce3+, Yb3+ = Colorless
Pr3+, Tm3+ = Green
Nd3+, Er3+ = Red
Pm3+, Ho3+ = Pink and Yellow respectively
Sm3+, Dm3+ = Yellow
Eu3+, Tb3+ = Pink
Gd3+ = Colorless
- Magnetic moment: Except La3+and Lu3+, all are paramagnetic due to unpaired f electrons.
- Ionization enthalpy: They have low ionization enthalpy.
- Electrode potential: Electrode potential value of these lanthanoids varies from –2.2 to –2.4V. There is one exception that is europium (electrode potential value = -2.0V).
- Complex formation: They have very less tendency to form complex because of very low charge size ratio.
- Chemical reactivity: All the lanthanides are highly electropositive metals and have almost similar chemical reactivity. The lanthanides are more reactive than d-block elements.
Chemical Properties of Lanthanides
There are some chemical properties of lanthanides:
- When these metals react with acids, they release hydrogen gas.
2Ln + 6HCl → 2LnCl3 + 3H2 - When Ln reacts with base, they liberate hydrogen gas. Their basicity decreases as the atomic number increases.
- These lanthanoids reacts with water, they form hydroxides and release hydrogen gas.
2Ln + 3H2O → 2Ln(OH)3 + H2 - When they react with nitrogen to form nitrides (LnN).
Ln + 1/2N2 → LnN - When they react with O2, they form oxides (Ln2O3).
- When lanthanides react with carbon on heating, they form carbides (Ln2C3).
- They react with halogens on heating and form halides (LnX3).
- They generally show +3 oxidation state. because +3 is more stable state.
Uses of Lanthanides
The uses of the compounds of lanthanides can broadly be classified follows:
- Non-nuclear applications
- Nuclear applications
- Ceramic applications: Ceria (CeO2), La2O3, Nd2O3 are widely used for decolorizing glass. About 1% CeO2 is used in the production of clear, transparent glass blocks for use in nuclear technology, as these blocks are not exposed to radiation for a long time. Adding more than 1% CeO2 to the glass gives it a brown color.
- Nd2O3 and Pr2O3 gives red and green colors respectively.
- Cerium salt is used in salt analysis, dyeing, lead accumulators, medicines.
- Cerium phosphate (CePO4) is used as catalyst in petroleum cracking.
- Lanthanide oxides are used for polishing glass.
- Ceria (Cerium(IV) oxide) is used in gas mantles.
- La, Ce, Pr, Nd mixed with steel are used in cigarette lighters.
- Certain alloys of lanthanides are used as misch metals.
- Mischmetal is also used in small amounts as lighter flints.
- They are also used in removal of Sulphur and Oxygen impurities from system.
- Gadolinium is another compound found in the elements lanthanide and used in hospitals, especially in the diagnosis and diagnosis of cancerous tumors using MRI.
- During radioactive degradation of promethium, phosphor light is generated from the generating agent, which is then converted into electricity by a solar cell.
- Neodymium oxide (Nd2O3) and nitrate (NO3-) are used to accelerate chemical reactions in the polymerization process.
- Neodymium is one of the elements of didymium glass that is used to make Google-specific glasses for welding.
- These are the symptoms used in eye surgery, skin cancer treatment, and plastic surgery.
- Praseodymium is used along with other lanthanoid elements in carbon electrodes for studio lighting and highlighting.
Lanthanides Interesting facts
Here we mention some interesting facts about lanthanides.
- The Swedish scientist Karl Gustaf Mosander discovered lanthanum in 1839.
- A relatively pure sample of the element was not produced until 1923.
- This is the first member of a series of lanthanide periodic tables.
- Although lanthanum is a rare earth mineral – named for the more abundant minerals due to its relative abundance – it is a common element.
- Lanthanum has been used in a wide variety of applications since its discovery, including vacuum tubes, hydrogen alloys, medicine, and molecular biology.
- Lanthanum is now used as a component of nickel-metal hydride batteries, which are primarily used commercially in hybrid vehicles.
- The discovery of lanthanum by hot cerium nitrate salts was made possible by the fact that lanthanum is the strongest base among all trivalent lanthanides.