17 Cl (Chlorine Element)

It is a yellow- greenish gas, combining directly with all other nearly elements, However, it is not as extremely reactive as Fluorine.
1 part of water dissolves 3.10 parts of chlorine gas at 10°C, and only 1.77 part at 30°C.
Chlorine gas is two and a half times heavy as air, and has an intensely disagreeable (not pleasant) suffocating odor, and extremely poisonous.
In its solid & liquid form, it is a powerful bleaching, oxidizing, & disinfecting agent.



Identity
CAS Number: CAS7782-50-5
CID Number: CID24526
RTECS Number: RTECSFO2100000
CONTENT INDEX
Basic Properties of Chlorine
Pronunciation: KLohr-een
Appearance: Pale yellow-green gas
Mass Number: 35
Standard Atomic weight: 35.457, 35.446 g/mol
Atomic number (Z): 17
Electrons: 17
Protons: 17
Neutrons: 18
Period: 3
Group: 17
Block: p
Element category: Diatomic Non metal (Halogens)
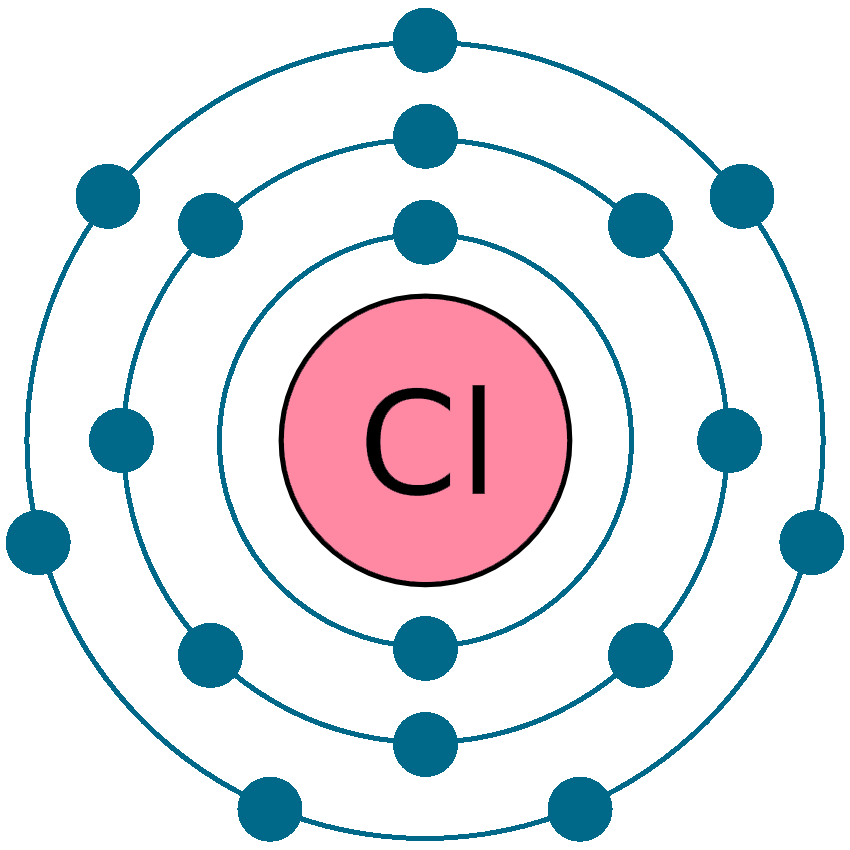
Electrons per shell: K2, L8, M7
Electron configuration: 1s22s22p63s23p5`
Thermal Properties of Chlorine
Phase: Gas
Melting point: 171.6 K (-101.5 oC, -150.7 oF)
Boiling point: 239.11 K (-34.04 oC, -29.27 oF)
Critical point: 416.9 K (143.75 oC, 290.75 oF)
Critical Pressure: 7.991 MPa (78.87 Atm)
Fusion heat: 6.406 kJ/mol (Cl2)
Vaporization heat: 20.41 kJ/mol (Cl2)
Specific heat: 478.2 J/(kg K)
Molar heat capacity: 33.950 J/(mol.K) (Cl2)
Thermal conductivity: 8.9×10-3 W/(m∙K)
Electrical properties of Chlorine
Electrical conductivity: 0.01×106 S/m
A Electrical resistivity: >10 Ω∙m
A Electrical type: Insulator
Magnetic Properties of Chlorine
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -40.5×10-6 cm3/mol
Volume magnetic susceptibility: -2.31×10-8
Mass magnetic susceptibility: -7.2×10-9 m3/kg
Molar magnetic susceptibility: -0.511×10-9 m3/mol
Physical Properties of Chlorine
Density: 3.2 g/cm3 (In STP) 1.562 g/cm3 (In Liquid at B.P)
Molar volume: 0.01103 m3/mol
Bulk modulus: 1.1 GPa
Sound Speed: 206 m/s
Atomic Properties of Chlorine
Oxidation states: 3, 6, 5, 4, 3, 2, 1, -1
Valence Electrons: 3s2 3p5
Ion charge: Cl–
Ionization potential of an atom: 12.96
Ionization energies: 1st: 1251 kJ.mol 2nd: 2298 kJ/mol 3rd: 3822 kJ/mol
Ionic radius: 1,81 pm
Atomic radius: 99 pm
Van der Waals: 175 Pm
Covalent radius: 102±4 pm
Filling Orbital: 3p5
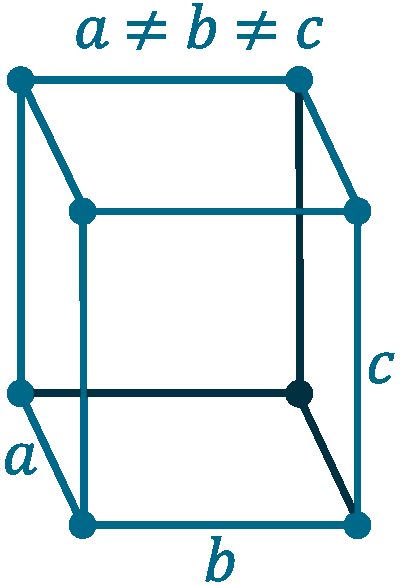
Crystal structure: Orthorhombic
Lattice angles: π/2, π/2, π/2
Lattice constant: 629.1, 450, 821 pm
Grid parameters: a=6.29 Å b=4.5 Å c=8.21 Å
Space Group Name: Cmca
Space Group Number: 64
Reactivity of Chlorine
Electronegativity: 3.16 (pauling scale)
Valence: +5
Electron affinity: 349 kJ/mol
Nuclear Properties of Chlorine
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2P3/2
Neutron cross section (Brans): 35.3
Neutron Mass Absorption: 0.033
Isotopes: 35Cl 36Cl 37Cl
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 35Cl | 76 | 34.969 | Stable |
| 36Cl | Trace | – | 3.01×105 y |
| 37Cl | 24 | 36.967 | Stable |
Chemical Reactions of Chlorine
It doesn’t react with oxygen & nitrogen, but reacts with carbon monoxide (CO), and forming COCl2:
Cl2 (g) + CO (g) → COCl2 (g)
When reacts with water, it forms hypochlorite (ClO–), where the position of equilibrium depends upon the pH of the solution.
Cl2 (g) + H2O (l) ⇌ OCl– (aq) + 2 H+ (aq) + Cl– (aq)
Fluorine Reacts with chlorine at 225 oC, and forming ClF & ClF3:
Cl2 (g) + F2 (g) → 2 ClF (g) (Chlorine (I) fluoride)
Cl2 (g) + 3 F2 (g) → 2 ClF3 (g) (Chlorine (III) fluoride)
Fluorine reacts at 225 oC & 225 Atm pressure, and forms ClF5:
Cl2 (g) + 5 F2 (g) → 2 ClF5 (g) (Chlorine (V) fluoride)
Bromine reacts with chlorine, and forming ClBr:
Cl2 (g) + Br2 (g) → 2 ClBr (g) (Bromine chloride)
Iodide reacts with chlorine, and forming ClI:
Cl2 (g) + I2 (g) → 2 ClI (s) (Chlorine iodide)
Reacts with hot aqueous alkali, and forming Chlorate (ClO3–).
3 Cl2 (g) + 6 OH– (aq) → ClO3– (aq) + 5 Cl– (aq) + 3 H2O (l)
Hydrogen reacts with Cl2 , and forming chloride, where the speed of reaction increase with increasing of temperature, even the reaction can be explosive under the right conditions.
H2 (g) + Cl2 (g) → 2 HCl (g)
Phosphorus reacts with excess Cl2, and forming phosphorus chloride:
P4 (s) + 10 Cl2 (g) → 4 PCl5 (s)
And reacts with excess phosphorus:
2 P (s) + 3 Cl2 (g) → 2 PCl3 (s)
PCl3 (s) + Cl2 (g) → PCl5 (s)
Sulfur reacts with excess Cl2, and forming sulfur (I) chloride or sulfur (II) chloride:
2 S (s) + Cl2 (g) → S2Cl2 (s)
S (s) + Cl2 (g) → SCl2 (s)
Cl2 reacts with So2, and forming:
SO2 (g) + F2 (g) → SO2F2 (g)
Cl2 reacts with H2S, and forming:
H2S (g) + Cl2 (g) → 2 HCl (g) + S (s)
Production
Chlorine is produced by electrolysis of sodium chloride (NaCl) dissolved in water, and gives chlorine with hydrogen gas & sodium hydroxide.
2 NaCl + 2 H2O (l) → Cl2 + H2 + 2 NaOH
Electrolysis of chloride solutions:
Anode:- 2 Cl– → Cl2 + 2e–
Cathode:- 2H2O + 2 e-‑ → H2 + 2 OH–
In Deacon process, Hydrogen chloride gas is convert to chlorine gas, by oxidation using oxygen:
4HCl + O2 → 2 Cl2 + 2 H2O
Chlorine History
Naming: Greek: khlôros (green).
Recognized by: Humphry Davy (1808)
Discovery and first isolation: Carl Wilhelm Scheele (1774) in Uppsala (Sweden)
Chlorine Uses
Chlorine is a disinfectant, that kills bacteria.
It is used to treat drinking & swimming pool water, and also used to make hundreds of consumer products, such as production of paper products, textiles, dyestuffs, petroleum products, medicines, antiseptics, insecticides, solvents, food, plastics, paints, etc…
About 20% of chlorine is used to make PVC (polymerization of vinyl chloride), which is very versatile plastic that used in window frames, electrical wiring insulation, water pipes, car interiors, blood bags, & vinyl flooring.
Most of the chlorine is used in the manufacture of chlorinated compounds for sanitation, pulp bleaching (for paper making & also used in industrially to remove ink from recycle paper), disinfectants, and textile processing.
Another major use of chlorine is in organic chemistry, where It is used in substitution reactions (single substitution reaction) & as an oxidising agent.
It is also used in the production of Chloroform (CHCl3, Sweet-smelling organic compound), Chlorates (ClO3–) , carbon tetrachloride (a dry-cleaning solvent, CCl4), & in the bromine extraction.
Chlorine gas is very poisonous, and it was used as a war gas (chemical weapon) during the First World War.
Biological role of Chlorine
Chloride ion is essential for life, and It is mostly present in cell fluid as a negative ‘-‘ ion to balance the positive ‘+’ ions (mainly potassium, K).
It is also present in extra-cellular fluid (all body fluid outside the cells, eg: blood) to balance the positive ions (mainly sodium, Na).
We get most of the chloride from salt, Typically we intake salt daily (about 6 gram), but we could manage with half this amount.
Chlorine is a respiratory irritant (slight inflammation or other discomfort), where the gas irritates the mucus membranes & the liquid burns the skin.
Exposure for chlorine should not exceed 0.5 ppm (8 hour time – 40 hour).
As little as 3.5 ppm (part per million) can be detected as an odour, and 1000 ppm is likely to be fatal (causing death) after a few deep breaths.
Abundance of Chlorine
Chlorine is found in nature in form of combined state only, where Halite (sodium chloride, NaCl) is the main mineral that is mined for chlorine.
NaCl is soluble salt that has been leached into the oceans.
Several salt lakes are found, where water has evaporated, which can be mined for chloride.
Chlorine is also found in the mineral Carnallite (hydrated magnesium potassium chloride, KCl.MgCl2·6(H2O)) and Sylvite (potassium chloride, KCl).
Chlorine gas are made by electrolysis process of brine (sodium chloride solution, NaCl), this process also produces useful sodium hydroxide (NaOH).
Annual world wide production is around 60 million tonnes.
0.0001% (In Universe)
0.037% (In Meteorites)
0.0008% (In Sun)
0.017% (In Earth’s Crust)
2% (In Oceans)
0.12% (In Humans)



World’s Top 3 producers of Chlorine
1) China
2) India
3) USA
Chlorine Price
Pure element price is around $1-$2 per KG (KiloGram)
#Chlorine


