76 Os (Osmium)

Osmium metal is a bluish white, lustrous, extremely hard, and brittle even at high temperatures.
It has the lowest vapor pressure and the highest melting point of the platinum group.
It is very densest metal, even little more than iridium (22.56 g/cm3).
The metal is difficult to fabricate, but the spongy or powdered metal reacts slowly with the oxygen and gives off osmium tetroxide (OsO4), which is a powerful oxidizing agent & has a strong smell, even it is highly toxic, and boils at 130 oC.
Osmium metal is uneffected by water and acids, but dissolves with molten alkalis.

Identity
CAS Number: CAS7440-04-2
CID Number: CID23937
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSRN1100000
CONTENT INDEX
Basic Properties of Zirconium
Pronunciation: Oz-mee-am
Appearance: Silvery, blue cast
Mass Number: 190
Standard Atomic weight: 190.23 g/mol
Atomic number (Z): 76
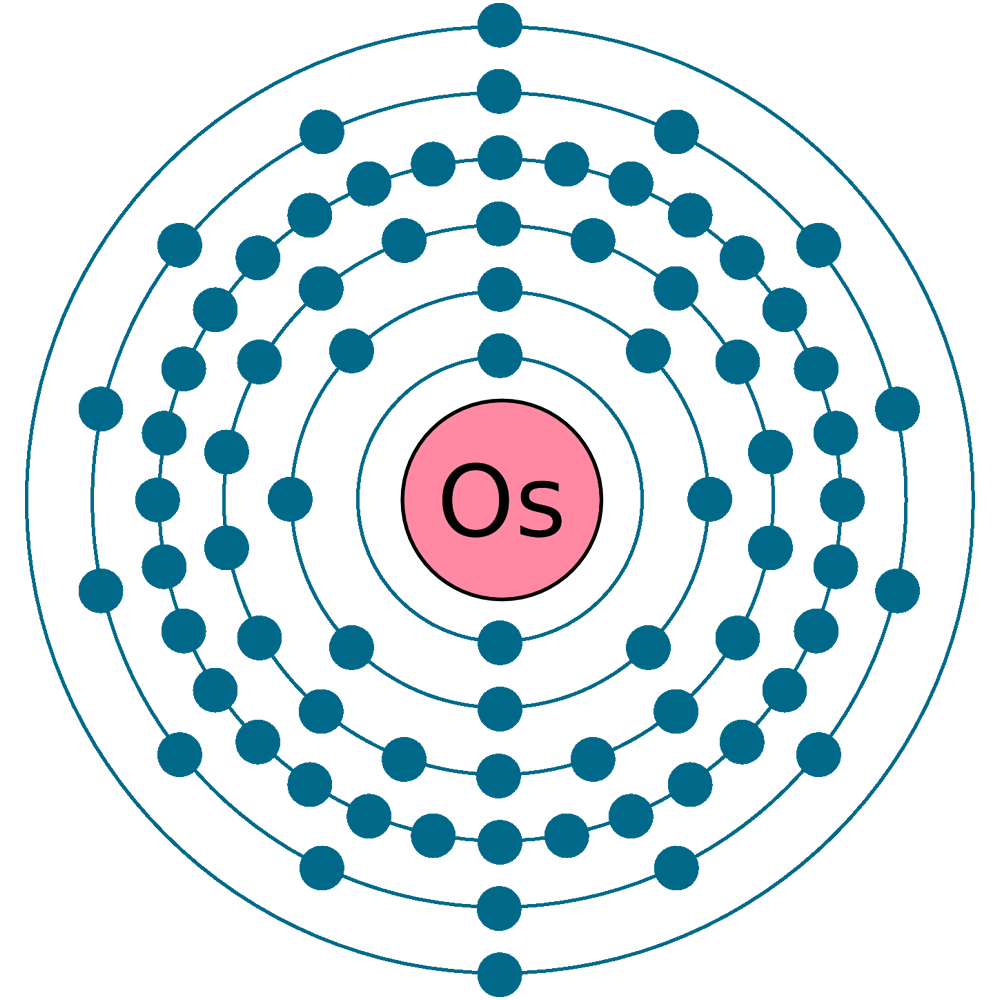
Electrons: 76
Protons: 76
Neutrons: 114
Period: 6
Group: 8
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N32, O14, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d66s2
Thermal Properties of Osmium
Phase: Solid
Melting point: 3306 K (3033 oC, 5491 oF)
Boiling point: 5285 K (5012 oC, 9054 oF)
Fusion heat: 31 kJ/mol
Vaporization heat: 378 kJ/mol
Specific heat: 130 J/(kg K)
Molar heat capacity: 24.7 J/(mol.K)
Thermal expansion: 5.1 μm/(m∙K)
Thermal conductivity: 87.6 W/(m∙K)
Electrical properties of Osmium
Electrical conductivity: 12×106 S/m
A Electrical resistivity: 81.2 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.66 K (-272.49 oC, -458.48 o F)
Magnetic Properties of Osmium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +11×10-6 cm3/mol
Volume magnetic susceptibility: 0.000014
Mass magnetic susceptibility: 0.6×10-9 m3/kg
Molar magnetic susceptibility: 0.11×10-9 m3/mol
Physical Properties of Osmium
Density: 22.59 g/cm3 (In solid) 20 g/cm3 (In Liquid at M.P)
Molar volume: 0.00002045 m3/mol
Shear modulus: 222 GPa
Mohs Hardness: 7.0
Bulk modulus: 462 GPa
Poisson ratio: 0.25
Vicker hardness: 300 MPa
Brinell hardness: 294 MPa
Sound Speed: 4940 m/s
Atomic Properties of Osmium
Oxidation states: -4, -2, -1, 0, 1, 2, 3, 4, 5, 6, 7, 8
Valence Electrons: 5d6 6s2
Ion charge: Os4+
Ionization energies: 1st: 840 kJ.mol 2nd: 1600 kJ/mol
Ionic radius: 63 pm
Atomic radius: 135 pm (empirical)
Van der Waals: 216 Pm
Covalent radius: 144±4 pm
Filling Orbital: 5d6
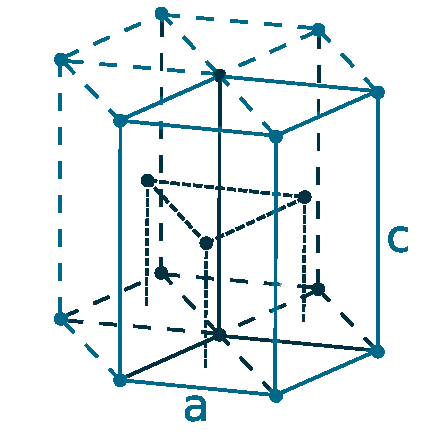
Crystal structure: Hexagonal close packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 273.4, 273.4, 431.7 pm
Grid parameters: a=2.734 Å c=4.317 Å
Attitude c/a: 1.579
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Osmium
Electronegativity: 2.2 (pauling scale)
Valence: +6
Electron affinity: 106.1 kJ/mol
Nuclear Properties of Osmium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 5D4
Neutron cross section (Brans): 15
Neutron Mass Absorption: 0.0023
Isotopes: 184Os 185Os 186Os 187Os 188Os 189Os 190Os 191Os 192Os 193Os 194Os
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 184Os | 0.02 | 183.953 | Stable |
| 185Os | Syn | – | 93.6 d |
| 186Os | 1.59 | 185.952 | 2×1015 y |
| 187Os | 1.96 | 186.955 | Stable |
| 188Os | 13.24 | 187.958 | Stable |
| 189Os | 16.15 | 188.954 | Stable |
| 190Os | 26.26 | 189.959 | Stable |
| 191Os | Syn | – | 15.4 d |
| 192Os | 40.78 | 191.961 | Stable |
| 193Os | Syn | – | 30.11 d |
| 194Os | Syn | – | 6 y |
Chemical Reactions of Osmium
The metal doesn’t react with air under normal condition, But when heated, it forms Osmium tetroxide (OSO4):
Os (s) + 2 O2 (g) → OsO2 (s)
Reacts with water, But not in normal condition:
Os (s) 2 H2O (l) ⇌ OsO2 (s) + 4 H+ (aq) + 4 e– Eo = -0.687 V
Os (s) + 4 H2O (l) ⇌ OsO42- (aq) + 8 H+ (aq) + 6 e– Eo = -0.994 V
Os (s) + 4 H2O (l) ⇌ OsO4 + 8 H+ (aq) + 8 e– Eo = -0.85 V
It Reacts with Fluorine under pressure & elevated temp., at 600 oC & 400 Atmosphere pressure to form Osmium (VII) fluoride, and under lower temp. & pressure, It forms Osmium (VI) fluoride:
2 Os (s) + 7 F2 (g) → 2 OsF7 (s) [yellow]
Os (s) + 3 F2 (g) → 2 OsF6 (s) [yellow]
For chlorine & bromine, under heat & pressure, forms the Osmium (IV) halides:
Os (s) + 2 Cl2 (g) → OsCl4 (s) [red]
Os (s) + 2 Br2 (g) → OsBr4 (s) [black]
Osmium History
Naming: Greek: osmê (odor)
Discovery & first isolation: Smithson Tennant (1803)
Osmium Uses
The metal is used to produced very hard alloyswith other metals of platinum group for phonograph needles, instrument pivots, high-quality fountain pen tips, and electrical contacts, and also used as a catalyst in the chemical industry.
Osmium tetroxide (OsO4) has been used to detect fingerprints and to stain fatty tissue for microscope slides.

Biological role of Osmium
The metal is not toxic & harmless, But finely divided metallic osmium is pyrophoric (ignites spontaneously in air below 55 oC), and its oxide is volatile and very toxic, causing lung, skin and eye damage.
Abundance of Osmium
Osmium occurs in the mineral osmiridium (an alloy with iridium) and in platinum-bearing river sands in the Urals, South America, and North America, and also found in the nickel-bearing ores of Sudbury, Ontario region.
Commercially, Most osmium is obtained from the wastes of nickel refining.
Annual world wide production Osmium is around 130 KG (Kilogram).
3×10-7% (In Universe)
6.5×10-5% (In Meteorites)
0.000065% (In Sun)
1.8×10-7% (In Earth’s Crust)

World’s Top 3 producers of Osmium
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Osmium
1) South Africa
2) Russia
3) USA
Osmium Price
Pure (99.99%) metal powder price is around $32,000 per KG (KiloGram)
#Osmium