06 C (Carbon Element)

CONTENT INDEX
About Carbon Element
Elemental carbon exists in three allotropic crystalline forms: Diamond, Graphite, and Fullerines. And a fourth form is known as “white” carbon, Other forms with little crystallinity are vegetal carbon and black fume. Graphite is one of the softest known materials while diamond is one of the hardest. “White” carbon is a transparent birefringent material.


Physical and Chemical properties of carbon depend on the crystalline structure of the element.
Graphite exists in two forms: Alpha (α) and Beta (β), where Naturally occurring graphites are contain as much as 30% of the Rhombohedral (beta) form, whereas synthetic materials contain only the alpha form. The hexagonal Alpha can be converted to the Beta by mechanical treatment, and when heating it above the 1000°C beta form reverts to the Alpha.
Elemental carbon is an inert substance, which is insoluble in water, diluted acids and bases, as well as organic solvents.
At high temperatures carbon binds with oxygen to form carbon monoxide (CO) or dioxide (CO2).
With hot oxidizing agents [Potassium nitrate (KNO3) and Nitric acid (HNO3)], Metilic acid C6(CO2H)6 is obtained.
Among the halogens only fluorine (F) reacts with carbon.
At ambient temperature (Room temperature) carbon tetrafluoride (CF4) is gas, tetrachloride (CCl4) is liquid and the other two compounds are solids.
Identity
CAS Number: CAS7782-42-5 (graphite), CAS7782-40-3 (diamond)
CID Number: CID5462310
DOT Hazard Class: 4.2
DOT Number: 1361
RTECS Number: RTECSHL4158550
Properties of Carbon
Basic Properties of Carbon
Pronunciation: car.ban
Allotropes: Graphite, Diamond, others (Fullerene, Carbon Nanotube, Graphene, Lonsdaleite, Amorphous Carbon)
Appearance: Black (graphite), Colorless / clear (Diamond)
Mass Number: 12
Standard Atomic weight: 12.011 g/mol
Atomic number (Z): 6
Electrons: 6
Protons: 6
Neutrons: 6
Period: 2
Group: 14
Block: p
Element category: Reactive Nonmetal
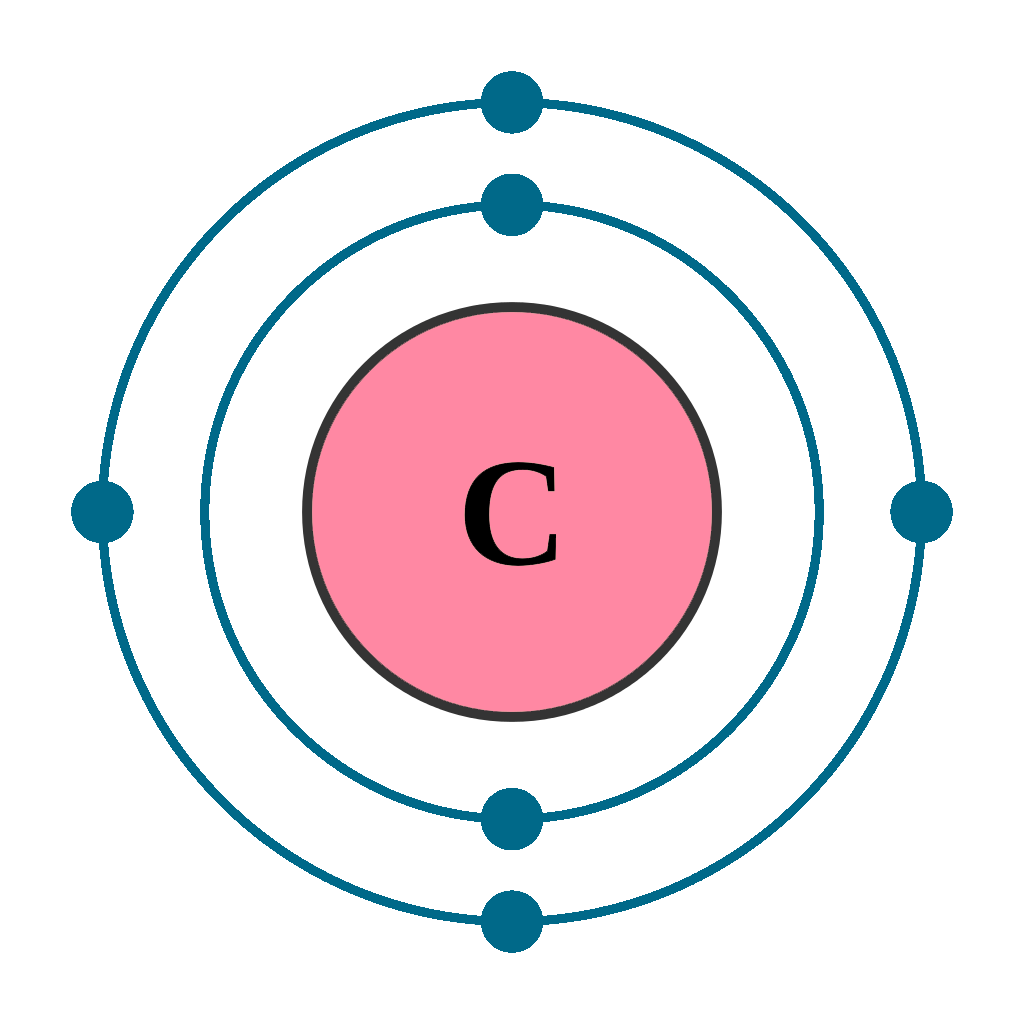
Electrons per shell: K2, L4
Electron configuration: 1s22s22p2
Thermal Properties of Carbon
Phase: Solid
Sublimation point: 3915 K (3642 oC, 6588 oF)
Melting point: 3823.15 K (3550 oC, 6422 oF)
Triple point: 4300.15 K (4027 oC, 7280.6 oF)
Triple point pressure: 10,800 KPa (106.58 Atm)
Debye temperature: K ( oC, oF)
Fusion heat: 117 kJ/mol (graphite)
Vaporization heat: 192 kJ/mol
Specific heat: 710 J/(kg K)
Molar heat capacity: 29.54 J/(mol.K)
Thermal expansion: 0.8 μm/(m∙K) (diamond)
Thermal conductivity: 120-165 W/(m∙K) (graphite), 900-2300 W/(m∙K) (diamond)
Electrical properties of Carbon
Electrical conductivity: 1×105 S/m
AElectrical resistivity: 7.836 μΩ∙m (graphite)
Electrical type: Conductor
Magnetic Properties of Carbon
Magnetic type: Diamagnetic
Curie point: N/A (above which Ferromagnetism vanishes)
Magnetic susceptibility (xmol): -5.9×10-6 cm3/mol
Volume magnetic susceptibility: -0.000014
Mass magnetic susceptibility: 6.2×10-9 m3/kg
Molar magnetic susceptibility: 0.0745×10-9 m3/mol
Physical Properties of Carbon
Density: 1.8-2.1 g/cm3 (amorphous), 2.267 g/cm3 (graphite), 3.515 g/cm3 (diamond)
Molar volume: 0.0000053146 m3/mol
Young’s modulus: 1050 GPa (diamond)
Shear modulus: 487 GPa (diamond)
Mohs Hardness: 1-2 (graphite), 10 (diamond)
Bulk modulus: 442 GPa (diamond)
Poisson ratio: 0.1 (diamond)
Sound Speed: 18,350 m/s (graphite)
Atomic Properties of Carbon
Oxidation states: -4, -3, -2, -1, 0, +1, +2, +3, +4
The ionization potential of an atom: 11.22 eV
Ionization energies: 1st: 1086.5 kJ.mol 2nd: 2352.5 kJ/mol 3rd: 4620.5 kJ/mol
Atomic radius: 77 pm (empirical)
Van der Waals: 170 Pm (picometre)
Covalent radius: 77 pm (sp3), 73 pm (sp2), 69 pm (sp)
Filling Orbital: 2p2
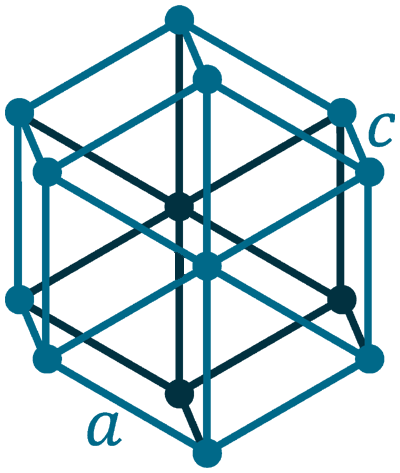
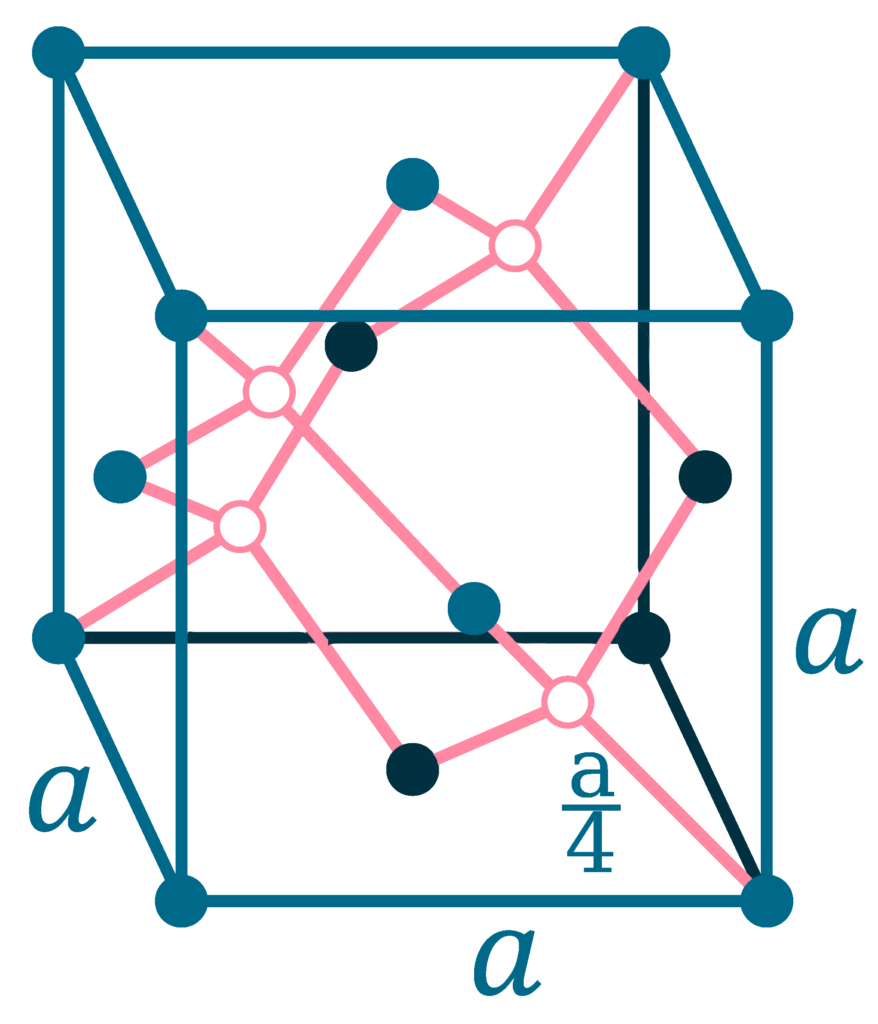
Crystal structure: Simple hexagonal (graphite), Face centered diamond cubic (diamond)
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 246.4, 246.4, 671.1 pm
Grid parameters: a=2.46 Å c=6.71 Å
Attitude c/a: 2.73
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Carbon
Electronegativity: 2.55 (pauling scale)
Valence: (+2), +4
Electron affinity: 153.9 kJ/mol
Nuclear Properties of Carbon
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P0
Neutron cross section (Brans): 0.0035
Neutron Mass Absorption: 0.000015
Isotopes: 11C 12C 13C 14C
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 11C | Syn | – | 20 min |
| 12C | 98.9 | 12.00 | Stable |
| 13C | 1.1 | 13.002 | Stable |
| 14C | Trace | 14.002 | 5730 y |
Chemical Reactions of Carbon Element
Reaction with Air
Carbon, as graphite burns and forming gaseous carbon oxide, even when Diamond heated to 600-800°C, it also burns and forming gaseous carbon oxides
At sufficient amount of oxygen (O2), carbon forms carbon dioxide (CO2), that is called complete combustion.
C (s) + O2 (g) → CO2 (g)
At insufficient amount of Oxygen (O2), carbon forms CO (carbon monoxide), that is called incomplete combustion.
2 C (s) + O2 (g) → 2 CO (g)
Reaction with water
It doesn’t react with water under normal condition. But At high temperature, carbon will react, when water is blown through hot coke. And forming a mixture of hydrogen (50%), carbon monoxide (40%), Nitrogen (5%) and Methane (5%).
2 C (s) + 3 H2O (g) → CO (g) + CO2 (g) + 3 H2 (g)
Reaction with Halogens
Graphite react with Fluorine (F2) at high temperatures, and forming a mixture of CF4, C2F6 + C5F12.
C (s) + F2 (g) → CF4 (g) + C2F6 + C5F12.
Reaction with Acids
Graphite reacts with the oxidizing acid hot concentrated Nitric acid (HNO3), and forms mellitic acid (C6(COOH)6).
Reaction with Calcium oxide
Carbon react with calcium oxide (CaO) at high temperatures, and forms calcium carbide (CAC2).
3 C (s) + CaO (s) → CaC2 (s) + CO (g)
Reaction with Sulfur
It reacts with sulfur at high temperatures, in the absence of oxygen, forming carbon disulfide (CS2).
C (s) + 2 S (s) → CS2 (g)
Reaction with Sodium amide
It Reacts with sodium amide at 500-600 °C, and forming Sodium cyanide (NaCN).
C (s) + NaNH2 (g) → NaCN (s) + H2 (l)
Carbon History
Naming: from the Latin word: carbo (charcoal).
Discovery: Egyptians and Sumerians (3750 BCE)
Recognized by (as an Element): Antoine Lavoisier (1789)
Carbon Uses
Carbon is unique among the elements, and it has the ability to form strongly bonded chains, sealed off by hydrogen atoms.
These hydrocarbons extracted naturally as fossil fuels (coal, oil and natural gas), which are mostly used as fuels. It is mainly used as feedstock for the petrochemical industries producing Fibres, Polymers, Paints, Plastics and solvents etc…
Impure carbon in the form of charcoal (from wood) and coke (from coal) is used in the iron and steel industries for Smelting process. Coal, charcoal and coke are amorphous forms of carbon is used as gas absorbent and bleaching agent.



Graphite (the purest form of carbon) is used in Pencils, to make Brushes in Electric motors, and in Furnace Linings. Activated charcoal is used for Purification and Filtration, that is found in Respirators (A device designed to protect the wearer from inhaling hazardous atmospheres) and Kitchen extractor hoods. Òther uses of graphite are in Dry cell and Light arch electrodes, and as a Lubricant.
Carbon fibre is the strongest material, that has a High Tensile Strength, Light Weight, High Stiffness, High Chemical resistance, High-Temperature Tolerance and Low Thermal Expansion. It is currently used in Skis, Tennis rackets, Fishing rods, Rockets and Aeroplanes.

Diamond (Free form or purest form of carbon) is used in Jewelry including decoration purposes.
Industrial diamonds are used as cutting tool for Cutting rocks and Drilling.
Diamond Films are used to protect surfaces such as Razor blades.
The more recent discovery of Carbon Nanotubes (CNTs), other Fullerenes and atom-thin sheets of Graphene has revolutionised hardware developments in the Electronics industry and in Nanotechnology.
Carbon Compound Uses
Atmospheric carbon dioxide (CO2) allows visible light but prevents some infrared escaping (the natural greenhouse effect), This keeps the Earth warm enough to sustain life. However, the enhanced greenhouse effect is underway, due to a human-induced (human-made disaster) rise in atmospheric carbon dioxide. which is affecting the living things by changes in our climate.
The temperature of climate increases with Increases CO2 in the atmosphere.
Other uses of free carbon element are as black fume pigment in Automobile’s rims and Printer’s ink.
Carbon compounds have many uses, Carbon dioxide (CO2) is used in drinks carbonatation (soft drinks etc..), in fire extinguishers and, in solid state as a cooling agent (dry ice).
(CO) Carbon monoxide is used as reduction agent (loses / donates electron) in many metallurgic processes.
Carbon tetrachloride (CCl2) and carbon disulphide (CS2) are important industrial solvents.
Freon (dichlorodifluoromethane, CCl2F2) is used in cooling systems.
Calcium carbide (CaC2) is used to prepare acetylene, which is used for welding and cutting metals, as well as for preparation of other organic compounds. Other metallic carbides have important uses as heat-resistants and metal cutters.
Carbon-11 is employed in medical diagnosis using the technique known as positron emission topography (PET).
Biological role of Carbon Element
Carbon element is more essential to life than other element, because only carbon forms strong single bonds (C-C) to itself that are stable enough to resist chemical attack under Ambient conditions. This give carbon the ability to form a huge variety of long chains and rings of atoms, which are the structural basis for many compounds that comprise the living cell, of which the most important is Deoxyribonucleic acid (DNA).
Living things get almost all their carbon from carbon dioxide (CO2), either from the atmosphere or dissolved in water. Photosynthesis by green plants and photosynthetic plankton (phytoplankton) uses energy from the sun to split water into oxygen and hydrogen, it consumes carbon dioxide, and release oxygen to the atmosphere, fresh water and seas, and the hydrogen joins with carbon dioxide to produce carbohydrates (CH2O) like Sugars, Starches and Fibers.
Some of the carbohydrates are used along with Phosphorus (P), Nitrogen (N) and other elements, to form the other monomer molecules of life, include bases and sugars for Ribonucleic acid (RNA) and Deoxyribonucleic acid (DNA), and Amino acids for proteins.
Living things that do not photosynthesise, they have to depend on consuming other living things for their source of carbon molecules. Their digestive systems break carbohydrates into monomers (A molecule that can be bonded to other identical molecules to form a Polymer), that they can use to build their own cellular structures.
Health Effect
Elemental carbon is very low toxic, but carbon black has very hazardous to health, and organizations presented health hazard data is based on exposures to carbon black, not on elemental carbon.
Chronic (persisting for a long time) inhalation exposure to carbon black may result in temporary or permanent damage to the Lungs and Heart.
Pneumoconiosis disease has been found in workers engaged in the production of carbon black.
Carbon black has been listed by the International Agency for Research on Cancer (IARC) within Group 3 (Unclassifiable as to carcinogenicity in humans).
Some Carbon compound can be very Toxic, such as Carbon Monoxide (CO) or Cyanide (CN-).
Carbon 14 is one of the radionuclides (An atom that has excess nuclear energy, making it unstable) involved in atmospheric testing of nuclear weapons. It is among the long-lived radionuclides that have produced. C-14 which is radioactive is used in “carbon dating” (telling how old something).
Most of what we eat (Fats, Carbohydrates, Proteins and Fibre) is made up of carbon compounds, giving us a total carbon intake of 300 gm/day. Digestion consists of breaking these compounds down into molecules that can be absorbed through the wall of the stomach or intestines. and they are transported by the blood to sites where they are utilized (some to replace or repair damaged cells, some to be turned into body messengers, and some to make the thousands of different chemicals that our body needs) or oxidised to release the energy they contain.
Abundance of Carbon Element
Carbon and its compounds are widely distributed in nature, It is found in the sun, stars, comets, and atmospheres of most planets (usually as carbon dioxide).
Carbon is found in the stars, formed from the debris of a previous supernova, which is built up by nuclear fusion in bigger stars.
The energy of the stars can be attributed at least in part to the well-known carbon-nitrogen cycle.
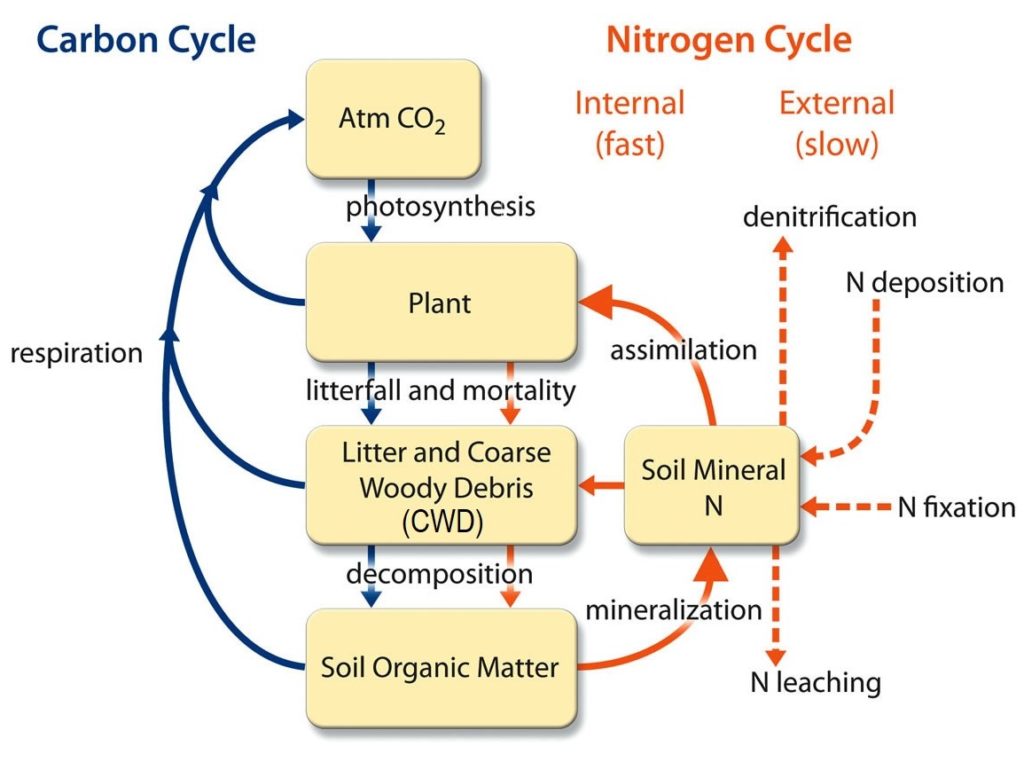
Currently concentration of carbon dioxide (CO2) on the earth atmosphere is around 414 ppm (part per million) and rising. Concentration of Carbon di-oxide (CO2) and Carbon monoxide (CO) is ever-increasing by cause of fossil fuel burning, and of methane (CH4) form paddy fields and cows.
The estimation is that carbon forms 0.032% of The Earth’s crust.
Free carbon is found in big reservoirs like hard coal (Anthracite), Amorphous (not crystalline) form of the element with other complex compounds of carbon(C)-hydrogen(H)-nitrogen(N).
All the Plants and live Animals are formed by complex organic compounds where carbon is combined with Carbon(C), Hydrogen(H), Nitrogen(N), Oxygen(O) and other elements.

Pure crystalline carbon is found in the form of Diamond, Graphite, and Fullerines. Carbon in the form of microscopic crystals (Diamonds) is found in some meteorites.
Natural diamonds are found in the mineral kimberlite of ancient volcanic pipes (narrow cone of solidified magma) found in South Africa, Arkansas, Russia, Botswana, DR Congo, Canada, and elsewhere.
Diamonds are now also being recovered from the ocean floor off the Cape of Good Hope (Africa).
About 30% of all industrial diamonds are now made of synthetically.

Graphite is found naturally in many locations.
In combination, Carbon is also found in all living things, and in nature as fossilized carbon in the form of hydrocarbons (natural gas, coal, crude oil, oil shales etc) and carbonates (chalk, dolomite, gypsum, limestone etc…).
Carbon Production
Chemically pure carbon can be prepared by Thermal decomposition of sugar (sucrose) in absence of air.
Fossile carbon (natural gas, coal, petroleum etc…) production is around 10 billion tonnes annually.
Annual world wide production of natural graphite is around 2 million tones.
Annual world wide production of mined diamond is around 165 million carats (33 tonnes), and synthetic diamond is around 4 billion carats (800 tonnes).
0.5% (In Universe)
1.5% (In Meteorites)
0.3% (In Sun)
0.18% (In Earth’s Crust)
0.0028% (In Oceans)
23% (In Humans)
World’s Top 3 producers of Coal
1) China
2) USA
3) India
World’s Top 3 Reserve holders of Coal
1) USA
2) Russia
3) China
World’s Top 3 producers of Diamond
1) Russia
2) Botswana
3) DRC
World’s Top 3 Reserve holders of Diamond
1) DRC
2) Botswana
3) Australia
World’s Top 3 producers of Graphite
1) China
2) India
3) Brazil
World’s Top 3 Reserve holders of Graphite
1) China
2) India
3) Mexico
Carbon Price
Pure Carbon (99.9999%) element price is around $10-$14 per Gram.
Coal Price is around $60 – $70 Metric tons (1000 KG).
Pure Graphite (99.9%) price is around $7000-$15000 per tonne (1000 KG)
Diamond price is around $17,000 – $20,000 per Carat (Carat is refer as weigh), Kohinoor Diamond is of 105 carat and its price is around $144,568,966 (10 Billion)
You Can Buy Pure Carbon Element and Carbon Products
Carbon Element Database
Atomic Spectroscopic Data of Carbon
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data of Carbon
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy of Carbon
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data of Carbon
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data of Carbon
→ Electrons
→ Helium Ions
→ Protons
Nuclear Physics Data of Carbon
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements



